Rice University hosted the 10th Annual Texas Life Science Forum this past week. The event was hosted by Ann Tanabe, Chief Executive Officer, BioHouston, and Brad Burke, Managing Director, Rice Alliance for Technology and Entrepreneurship, representing the two groups organizing the event.

Keynote by Stephen M. Hahn M.D., former Commissioner of Food and Drugs, U.S. Food and Drug Administration overseeing the Operation Warp Speed Covid vaccine approval process.
As head of the FDA during the Trump administration, Stephen M. Hahn M.D. oversaw the rapid approval process of the mRNA Covid vaccines as part of Operation Warp Speed as well as the FDA’s Pandemic Recovery and Preparedness (PREPP) Initiative.
Hahn is clearly a man on the move, a figure who is championing the idea of applying the lessons learned during the Covid vaccine development process to other healthcare issues.
Hahn pointed out that from the date that Chinese researchers first sequenced the Covid-19 virus (January 10, 2020), it took only 11 months for the FDA to issue its initial emergency use authorization for the Pfizer vaccine – the previous record for vaccine approval was four years for the Ebola vaccine.
Now at Harbinger Health (a Flagship Pioneering Company), Hahn is promoting the principles of faster drug approvals using real-world data. He also defended the controversial decision during his tenure at the FDA to approve Biogen’s Aducanumab drug for Alzheimer’s Disease, saying that if real-world data proves it is ineffective, then FDA approval could be revoked at a future date.
He also emphasized we face an urgent need to shift from a “sick care” paradigm to a pre-emptive medicine paradigm – to address the other “silent pandemics” we are facing.
These include:
- Antimicrobial resistance, aka AMR (69 million potentially affected)
- Paramyxovirus (incl smallpox) (400 million potentially affected)
- Ischemic heart disease (204 million potentially affected)
- Diabetes (44 million potentially affected)
- Stroke (115 million potentially affected)
- Alzheimer’s (82 million potentially affected)
- Prostate cancer (13 million)
- COPD (73 million)
- Depression (suicide) (18 million)
- Drug addiction (5 million)
To achieve this, Hahn sees Artificial Intelligence and Machine Learning* as the key technology for developing new predisease prediction and intervention protocols, allowing healthcare clinicians to address disease while it’s still reversible before chronic dysregulations and imbalances occur.
(See the new FDA AI/ML guidelines issued during Hahn’s leadership of the FDA here.)

Major Life Science Initiatives along the “Third Coast”
Today the biggest life science research clusters are found on the east coast (in Boston and the greater NYC area) and on the west coast (e.g. in San Francisco and San Diego).
But what about the “Third Coast” along the Gulf of Mexico?
Houston already boasts the world’s largest single concentration of healthcare facilities in the world – at the Texas Medical Center, or TMC – so this begs the question: why couldn’t this be a launchpad for another major life science research cluster?
That’s the question on the minds of life science leaders in Houston and across the Southwest, and at the forum, we got an idea of the scale of investment that is currently underway to make it happen.
Texas Medical Center: Life Science Vision for Houston – presentation by Isaac Middleton, COO, Texas Medical Center
According to Isaac Middleton, the COO of the Texas Medical Center, the key initiative to making Houston the center of a new life science research cluster is the TMC3 initiative, a 37 acre, $5.5 billion project which broke ground in the fall of 2021.
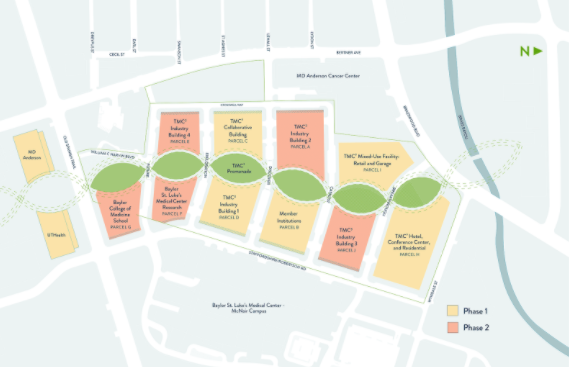
When completed, the TMC3 will comprise 6 million square feet of office, laboratory, residential, and hospitality space.
Phase One of the project will be anchored by a 700,000 sq ft facility developed by Boston-based Beacon Capital in partnership with the life science investment firm Braidwell. This phase will also include a separate 500 room hotel with 65,000 sq ft of conference space and a residential tower with 350 units.

Middleton said the philosophy behind the TMC3 initiative revolves around bringing in private for-profit industry partners directly onto the campus, something that has never been done before (it actually required changing some of the legal covenants on the real estate, he explained.)
TMC is also entering the venture capital funding space, with its new TMC Ventures Fund, with an initial $25 million available to invest in promising new startups and technologies.
TMC BioBridges: A Global Destination for Healthcare Commercialization – presentation by Tom Luby, Ph.D., Director, TMC Innovation
Creating a broader and deeper life science ecosystem is the mission of TMCi, the Medical Center’s innovation startup center. Located in a spacious repurposed former Nabisco cookie factory, the TMCi hosts a new cohort of early-stage companies each year, offering them office space, mentoring services, and more.
But according to Tom Luby, Ph.D., Director, TMC Innovation, there is more than can be done to accelerate the number of life science companies in the region and create a larger biotech ecosystem.
One such initiative, called TMC BioBridges, seeks to make Houston and the TMC the preferred destination for overseas life science companies seeking to open US markets.
To date, the TMC BioBridges program has inked partnerships with the UK, Denmark, and Australia to facilitate a “white glove” program to provide all the necessary services for these companies to open new operations in the US with assistance from the Texas Medical Center.

JLABS @ TMC: What’s New? – Presentation by Fiona Mack, Ph.D., Head of JLABS TMC, Johnson & Johnson Innovation
Johnson & Johnson is also a partner with the Texas Medical Center; it opened one of its JLAB innovation facilities on the TMCi campus in 2016, one of 13 across the globe.
Fiona Mack, Ph.D. is the head of the Houston JLABS, and she outlined how the company is streamlining its operations worldwide to encourage startup companies participating in the different JLAB locations to work more closely together. J&J also plans to offer more early-stage equity investments to its members to speed up innovation within the parent company’s drug and medical device pipeline.
Mack points out that JLABS @ TMC has already helped over 100 companies, which have collectively raised over $3.4 billion in investment to date.
Mack also identified some important new non-dilution grant funding opportunities as part of JLABS’ new “Blue Knight” partnership with the federal government’s Biomedical Advanced Research and Development Authority (BARDA).
MD Anderson’s Strategy for Accelerating Cell Development – Jason Bock, Ph.D., Vice President & Head of Biologics Product Development, MD Anderson
The MD Anderson hospital at the Texas Medical Center is world-renown for its leading-edge cancer care.
At the forum, Jason Bock, Ph.D., the Vice President & Head of Biologics Product Development at MD Anderson, gave an important overview of the latest developments in cell therapy for cancer treatment, including Chimeric antigen receptor (CAR) T-cell therapy.

This approach involves extracting T-cells from a cancer patient’s blood and sending them to a lab where they are genetically modified to produce a “receptor” protein that helps the cell recognize antigens on “bad” cancer cells. The modified T-cells are reinjected into the bloodstream of the patient, where they can fight off the disease more effectively.
But the question, according to Bock, is where should this vital lab work take place? He said the team at MD Anderson realized that they needed to invest in a new lab facility.
This new thinking led to the development of the nearby Cell Therapy Manufacturing Facility, a 60,000 sq ft complex, which includes 14 GMP clean rooms and supporting QC labs, as well as a 3,500 sq ft cGMP area for viral vector manufacturing.
This investment, says Boyd, will help MD Anderson support new disease treatment modalities beyond CAR-T, including TIL, Allogeneic T Cells, and Endogenous T Cells, as well as GMP-grade viral vector production using Retrovirus and Lentivirus based delivery mechanisms.

The Ten Most Promising Life Science Companies
Throughout the conference, participating companies made elevator pitches to the audience, which included clinical providers, researchers, medical administrators, as well as entrepreneurs and VC investors.
After careful consideration by the team at the Texas Life Science Forum awarded ten prizes to the most promising life science companies:
1. Ares Immunotherapy
Ares Immunotherapy is developing a candidate chimeric antigen receptor (CAR) T cell that targets mesothelin, which in animal studies has shown a significant improvement in solid tumor regression.
2. Corveus Medical (formerly Caridian)
Corveus Medical has developed a new catheter-based device to perform targeted sympathetic nerve ablation to treat heart failure.
3. Drusolv Therapeutics
Drusolv Therapeutics has developed a novel reformulation of the statin drug Atorvastatin to treat patients suffering from early-stage AMD (the leading cause of blindness) to prevent progression to late-stage vision loss.
4. EMPIRI
Empiri is developing a unique clinical approach to oncology treatment by extracting tumor cell tissues from a cancer patient then slicing them into separate cultures to identify the best treatment method for that specific tumor, whether it’s chemotherapy, targeted cell therapy, an immunotherapy, or a combination thereof.
5. Lapovations
Lapovations has created a single-use medical device to assist laparoscopic surgeons with lifting the abdominal wall prior to insertion, thus avoiding injury by conventional methods, such as towel clips.
6. Maxwell Biosciences
Maxwell Biosciences is developing non-peptide small molecules that mimic the function of bioactive human peptides, particularly cathelicidin antimicrobial peptides, which are among the most effective molecules of the human innate immune system.
7. NeuraStasis
NeuraStatis is developing a system to preserve brain tissue immediately after an ischemic stroke by putting neural tissue into stasis so that major organs can do a better job surviving with low oxygen, allowing providers precious time to administer more long-term treatment.
8. Vena Medical
Vena Medical is developing the world’s smallest camera for doctors to see inside veins and arteries as part of stroke treatment.
9. Vivifi Medical
Vivifi Medical is developing new methods to treat male prostate gland enlargement, known as Benign prostatic hyperplasia (BPH). The company seeks to create a solution that is less painful and offers a quicker recovery time, while retaining sexual function, compared to the conventional TURP procedure.
10. XN Health
XN Health is developing a new approach to phrenic nerve stimulation for patients requiring mechanical ventilation and prone to a condition known as ventilator induced diaphragm dysfunction (VIDD), a loss of respiratory strength that makes recovery slower.
Micheal E. DeBakey Award
But wait! There’s more.
Named in honor of Michael E. DeBakey, the famed cardiovascular surgeon at the Texas Medical Center whose career spanned 75 years, this award is bestowed on the company with the most promising innovation in cardiac surgery.
This year’s Micheal E. DeBakey Award winner is the Austin-based company Dynamic Light.
Dynamic Light
Dynamic Light is a real-time video system that provides cardiovascular surgeons with an enhanced, full-color, continuous view of the patient’s circulatory blood system at the operating table. It uses a non-invasive camera that can identify active blood vessels which are hard to differentiate with the naked eye, allowing surgeons instant feedback on where to avoid clipping or cutting to prevent blood loss. This system replaces potentially harmful and costly pre-surgical radioactive dye injections.

Let’s Build the Future of Life Science Together
If you can imagine it, we can build it, here at our factory headquarters in Austin, Texas.
Formaspace manufacturers high-quality laboratory furniture for your research laboratories, clinical testing labs, GMP manufacturing facilities, and more.
Find out how we can make your next lab project a success.
Contact your Formaspace Design Consultant today.












