While overall cancer survival rates have been edging up in recent decades, cancers in the lungs have generally remained more difficult to treat successfully.
But this month, there was good news for patients diagnosed with Non-Small Cell Lung Cancer (NSCLC) who harbor the T790M genetic mutation. According to a new article published in the New England Journal of Medicine, AstraZeneca’s drug Osimertinib (marketed under the brand name Tagrisso) can increase the 5-year overall survival rate to 88% among patients with the T790M mutation who have undergone lung surgery to remove NSCLC tumors.

Statistically speaking, this is really good news, but it’s also not immediately obvious exactly which patients can benefit from this clinical therapy.
To understand the scope of the problem, let’s look at relevant statistics for the different types of lung cancer and associated genetic mutations.
Types of Lung Cancers and Associated Genetic Mutations
The topline statistic is very sobering: lung cancer is a leading cause of cancer death in the US.
According to the American Cancer Society, incidences of lung cancer diagnoses rank just behind breast cancer for women and prostate cancer for men. While it’s not the most common form of cancer (according to the CDC, skin cancer, breast cancer, and prostate cancer are the most common, followed by lung cancer), lung cancer remains by far the deadliest – it is responsible for 1 in 5 cancer deaths.
The American Cancer Society estimates that there will be about 240,000 new lung cancer diagnoses and about 130,000 deaths in 2023.
Demographically, lung cancer is more often diagnosed among older people; the average age of those diagnosed with the disease is 70.
Those with a history of smoking are affected most. Fortunately, the number of new cases is edging downward, a trend that appears to be associated with a broad societal reduction in smoking.
Small Cell versus Non-Small Cell Lung Cancer (NSCLC)
Oncologists generally divide lung cancer into two categories: small-cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC).

Tissue samples taken during a biopsy can reveal the difference. As the name implies, the size of individual cells associated with small-cell lung cancer is significantly smaller.
SCLC affects fewer patients overall, comprising only about 10-15% of cases. But it’s very aggressive and can spread quickly to many parts of the body, often before it’s diagnosed. SCLC is also more often associated with a history of smoking.
Non-small cell lung cancer (NSCLC) is the more common diagnosis, responsible for 80 – 85% of lung cancers. There are three major types of NSCLC:
· Adenocarcinomas
This is the most common type of lung cancer, responsible for 40% of all cases, and fortunately, it is the most treatable. Adenocarcinomas often form in the peripheral areas of the lung and can spread via the lymph nodes. Adenocarcinomas can be mistaken for a case of pneumonia during a chest X-ray as they often have a similar presentation.
· Squamous Cell Carcinomas
This type of lung cancer is responsible for 25-30% of cases (a lower percentage than in years past). Over time, a Squamous Cell Carcinoma can form a large cavity in the central bronchial area of the lungs.
· Large Cell Carcinomas
Also known as undifferentiated carcinomas, these are relatively uncommon compared to the other types – comprising only 10-15% of all lung cancer diagnoses. Unfortunately, however, Large Cell Carcinomas tend to spread via the lymph nodes to other parts of the body.
The Role of the T790M Genetic Mutation for Epidermal Growth Factor Receptor (EGFR) in Treating Non-Small Cell Lung Cancer (NSCLC)
The identification of the T790M genetic mutation in lung cancer patients is one of the more significant gene sequencing discoveries in oncology.
The T790M gene mutation, which is present in about one-quarter of patients worldwide, affects the function of the Epidermal Growth Factor Receptor (EGFR).
T790M essentially serves as a “master switch” for Epidermal Growth Factor Receptor by substituting a threonine amino acid in place of a methionine amino acid; this change can allow cancer cells to grow and spread rapidly throughout the body.
What is the specific mechanism that allows this to happen?
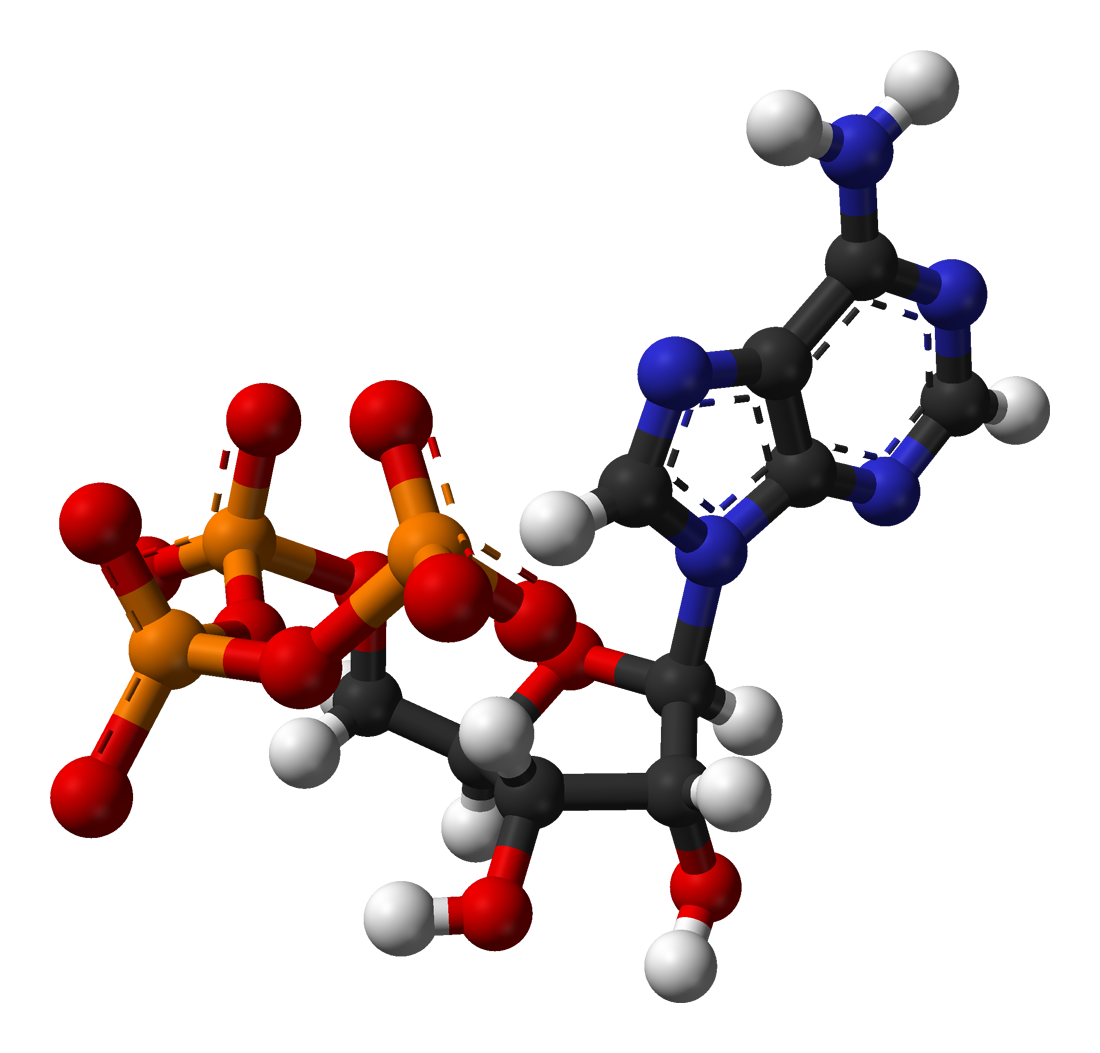
This threonine amino acid genetic mutation accelerates the ability of Adenosine triphosphate (ATP) – a major source of “growth energy” throughout the body – to bind to the Epidermal Growth Factor Receptor (EGFR) gene to such an extent that it outcompetes other “inhibitor” amino acids that try to put the brakes on unregulated cell growth.
In other words, with ATP on full throttle, applying the ‘brakes’ using inhibitors is not effective, allowing cancer cells to propagate quickly.
As we’ll see in a moment, overcoming this failure of the natural growth inhibitors to limit ATP binding is the key challenge for drug designers seeking to reverse the development of non-small cell lung cancers.

The Challenge of Developing a Drug to Treat Non-Small Cell Lung Cancer
Today, we can celebrate the results of the recent study that AstraZeneca’s drug Osimertinib (marketed under the brand name Tagrisso) that can help raise the 5-year survival rate among T790M gene mutation patients who have undergone lung surgery to remove NSCLC tumors to 88%.
But how did the lab researchers at AstraZeneca accomplish this goal?
The drug discovery program started back in 2009 with the intent of finding a third-generation, novel therapeutic compound that would address those patients with the T790M gene mutation. The need was great – when these lung cancer patients received conventional oncology therapy, their disease progression was worse after treatment.
It took 2 ½ years to identify a suitable new drug compound, which was named Osimertinib.
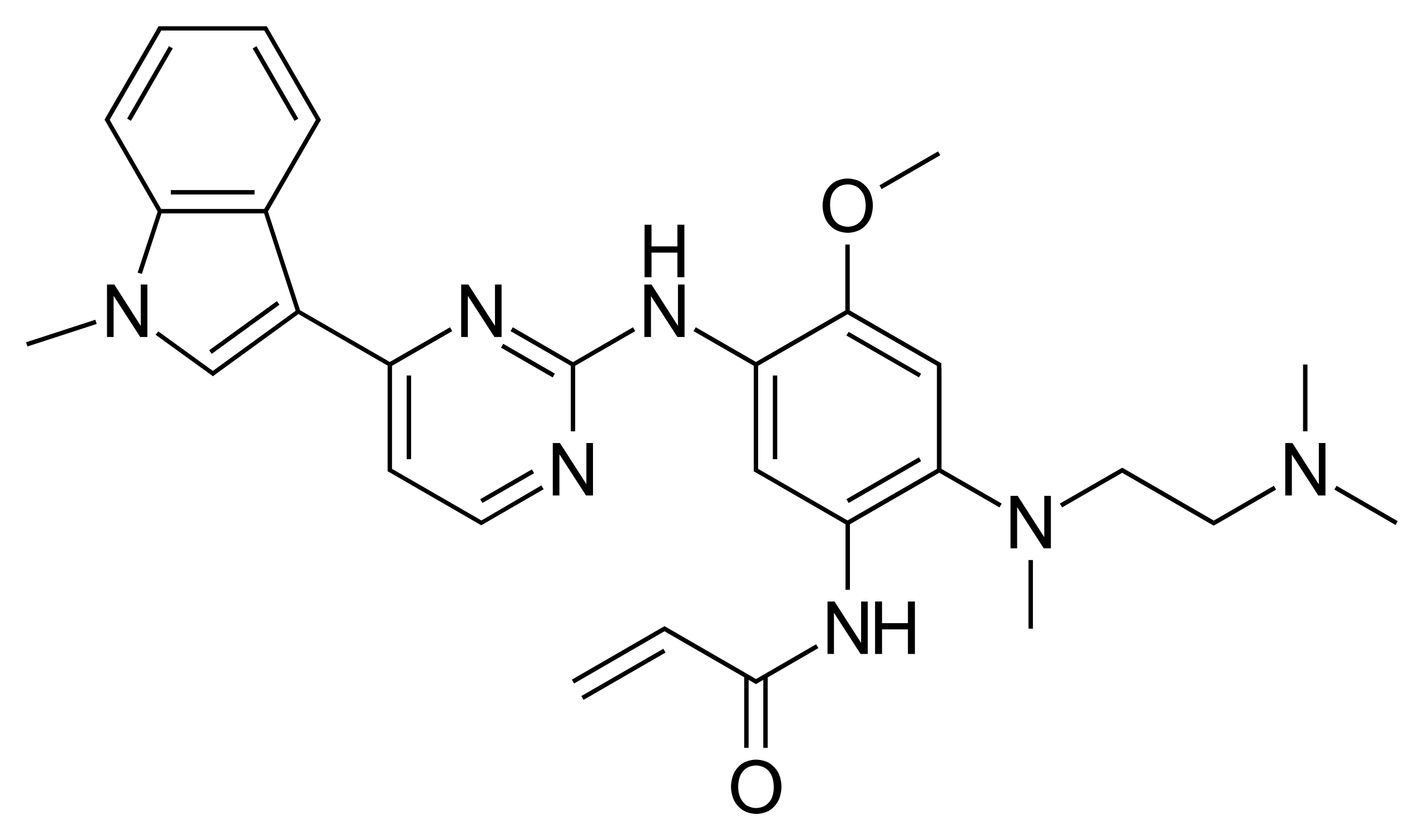
Three Generations of Drug Development for Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors (EGRF-TKIs)
- First Generation: Gefitinib and Erlotinib activated mutations in the EGFR gene.
- Second Generation: Afatinib and Dacomitinib offered more potent inhibitors but at the cost of increased toxicity.
- Third Generation: Osimertinib overcomes the increased ATP binding activity to EGFR affected by the T790M gene mutation without affecting wild-type EGFR, thus reducing toxicity.
Approval for the New Drug by the FDA Came Quickly; Osimertinib Received the first ever FDA “Fast Track” Approval
The FDA designated Osimertinib as part of its “Orphan Drug” program, which provides additional tax credits and patent protection to encourage the development of new drug therapies for relatively rare diseases.
In 2014, in a first for the agency, the FDA designated Osimertinib as a “Breakthrough Therapy,” which afforded it “Fast Track” priority in the drug approval process.
Thanks to the expedited process, the FDA awarded provision approval of Osimertinib in 2015 for NSCLC patients with the T790M gene mutation who were not responding to conventional therapy. The FDA also approved the necessary new clinical laboratory testing protocols to identify the positive presence of the T790M gene mutation among lung cancer patients.
In 2017, the FDA gave full approval for Osimertinib for patients with the T790M gene mutation.

Osimertinib Approved as a Post-Surgery “Adjuvant Therapy”
In 2020, Osimertinib became the first cancer drug approved by the FDA for use immediately after tumor removal, in what is called an “adjuvant therapy.”
From a medical perspective, the theory here is that Osimertinib can help prevent the recurrence of lung cancer after surgery – by blocking excess ATP binding to the epidermal growth factor receptor (EGFR) that would otherwise accelerate cell growth in patients with the T790M gene mutation.
While there are concerns that the body can develop long-term resistance to the Osimertinib therapy in some cases (if this occurs, it generally becomes an issue after 10 months), the results among those who had the T790M mutation were very encouraging:
According to the study published in the New England Journal of Medicine, the study of 682 patients with stage IB to IIIA disease, the 5-year overall survival rate increased to 88%.
This is significantly higher than statistics published by the American Cancer Association, which report the 5-year relative survival rate of Localized NSCLC (e.g. cancer tumors diagnosed within the lung but not spread to other organs) was only 65% between 2012 and 2018.
These outcomes are also far better than for untreated NSCLC patients. A 2013 study estimated that at that time, untreated lung cancer patients only lived on average for 7.15 months.
There is a financial cost associated with these better health outcomes, of course.
When AstraZeneca brought Osimertinib to market (under the brand name Tagrisso), it initially charged about $13,000 for 30 tablets (a month’s supply). Today, the retail price is closer to $17,000, which works out to just over $200,000 annually. Depending on circumstances, some patients may be prescribed the drug for as long as 3 years; in that case, the course of treatment for the drug alone would cost $612,000 at retail drug prices, although it’s assumed that insurance companies pay lower, unpublished rates.

Formaspace is Your Laboratory Research Partner
If you can imagine it, we can build it, here at our factory headquarters in Austin, Texas.
Take the next step. Talk to your Formaspace Design Consultant today and see how we can work together to make your next laboratory construction project or remodel a complete success.











