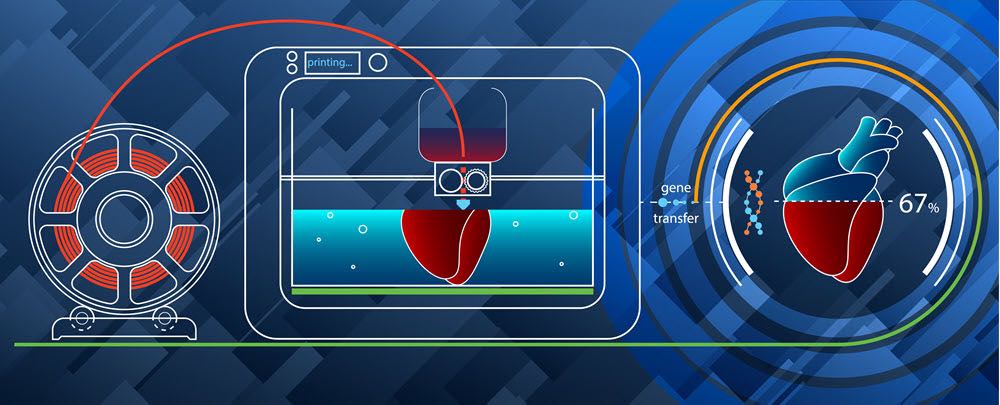
How Close Are We to 3D Printing Molecular Structures, such as Vaccines?
One of our readers recently asked: “if we can “3D print” human organs for scientific research, why can’t we also ‘print’ vaccines or other molecular structures?”
Indeed, since we last wrote about the possibilities of creating human organs on a 3D printer, researchers are making important advances at new startup companies, such as YCombinator’s Volumetric, as well as at NASA, which is planning to test the efficacy of “bioprinting” artificial organs in space.
Given that background, the question is entirely reasonable.
So, is it possible? Can we print molecular structures, such as vaccines, on a 3D printer?
The answer is that we are closer than you might think.
What Kind of Vaccines Can Be Designed on the Computer?
Let’s break this question down into its components, starting with the vaccine design process, and ask the question again.
Is it possible to design vaccines on the computer, much like a product designer would use a CAD program to design a new part to be made on a 3D printer?
The answer is a definite yes, and the millions of people around the world who received a double jab of Moderna or Pfizer/BioNTech Covid-19 vaccine are proof that the process works.
In fact, senior leaders from both Pfizer/BioNTech and Moderna have been quoted during interviews that one of the quickest steps in developing the new Covid-19 was the design process, which both companies claim they each completed record time after receiving the viral genomic code published by Chinese researchers on January 11, 2020.

How Can a New Vaccine Design Be Accomplished so Quickly?
How could the new mRNA Covid vaccines, which Moderna co-founder Derrick Rossi says at his company designed in just a couple of days, be developed so quickly?
Part of the reason comes from the inherent simplicity of an mRNA vaccine itself, which has a very important but uncomplicated job – it jolts the human body’s immune system in action by circulating replicas of features found in the real virus.
As these replicas circulate through the body, the immune system learns to recognize and attack the intruder, e.g. the vaccine, giving us immune protection against the real virus should we be become infected in the future.
What’s the Process for creating an mRNA Coronavirus Vaccine Design on the Computer?
So how do vaccine designers go about it?
The first decision that vaccine designers have to make is to determine which antigen feature of the live virus the human immune system needs to “learn” to provoke a strong immune response.
In the case of the coronavirus, the answer is obvious: it’s those distinctive “spikes” we’ve all become familiar with that are found on the outer envelope of the Covid 19 virus.
And, because these spikes are described in the virus’ genomic code, it’s a straightforward process for the vaccine designer to copy the genetic code of the spike structure to create a similar spike structure in the new mRNA vaccine.
Thus when the mRNA vaccine passes through the body, our immune system will learn to recognize this distinctive spike structure and attack all future invaders with this same unique feature, helping us achieve immunity against future infection.
The second decision for the vaccine designer is the selection of a delivery mechanism, which in this case is a fragile strand of messenger RNA (mRNA) encapsulated in a nanoparticle-sized lipid (fat) sphere.
Once it’s inside the body, it will, as mRNA does, replicate many copies of itself over a short period before it naturally decomposes and is flushed away.
Choosing an mRNA-based system has other advantages, as we’ll see shortly, including a much simpler manufacturing process that does not rely on any animal or plant cells for replication.

What about Designing Vaccines to Protect against Covid-19 Mutations, e.g. the Variants?
Unfortunately, with so many people around the world still not vaccinated, the virus has had a free pass to mutate since it was first discovered.
(TIP: see our report on how to encourage your employees to get vaccinated at work.)
So far, we’ve seen several variants emerge, including the Delta variant (which can shed 1,000 times more virus compared with the original virus) that most likely originated in India but is now spreading rapidly in the US, as well as the highly contagious Lambda variant that is currently the dominant strain in Peru but was also recently identified in the Houston area.
It’s also not out of the question that even more dangerous variants could yet emerge.
Fortunately, there is good news regarding designing new vaccines to combat these variants.
The very same design process which quickly created the initial vaccine can be used to design updated versions that attack these newer, more contagious variants.
Moderna and Pfizer/BioNTech are reportedly working behind the scenes to prepare booster shots that will also have updated designs that mimic the slightly different spike structures found in the new variants – to help the body’s immune system recognize them and provoke a strong immune response.

Can We Design Bigger, More Complex Molecules, such as Proteins, on the Computer?
Let’s take a quick detour and talk about designing much larger, more complicated molecules, such as proteins. There are about 200 million known proteins in nature, and their structures are much more complicated (and larger) than a typically small, simple strand of RNA.
Proteins also “fold” onto themselves, which is a complicated process that is incredibly hard to model on the computer.
Fortunately, this is where AI comes to the rescue. Researchers using Google’s DeepMind AI platform have been able to create a successive generation of AI-based tools, known as AlphaFold, that can solve these incredibly complex problems.
In late 2020, the newest version of the software, AlphaFold 2, won the 14th annual Critical Assessment of Techniques for Protein Structure Prediction (CASP) competition.
Can We Work Directly with Individual Atoms Yet?
But you might be saying, “Hey! Wait a minute!”
Work with representations of large molecular on the computer is not the same thing as working directly with individual atoms in real life.
So spoiler alert! It’s true. We can’t (yet) manipulate individual atoms and put them into place one by one to build a new molecule, as you would do with an analogous 3D printer, e.g. building a new part layer-by-layer.
In fact, not only is it hard to manipulate individual atoms, it’s actually hard to even see them… as lab managers well know, it takes complex equipment and sophisticated techniques (such as x-ray crystallography, cryo-electron microscopes, or even nuclear magnetic resonance equipment) to see the atomic structures inside complex molecules, such as proteins.

But the good news is that we have indirect tools to manipulate biomaterials.
But researchers are also becoming more versant in using biological processes to assemble molecules, for example, using the body’s own DNA and RNA replication processes.
These natural processes essentially mimic the output of a 3D printer by reading the design code and creating new copies.
We’ll discuss how this works in the case of creating an mRNA Covid vaccine in the next section.
Converting a Vaccine Designed on a Computer into a Prototype Drug for Clinical Testing
So how do you translate a vaccine designed on the computer into a real prototype drug design suitable for evaluation in clinical trials?
Here we will rely on the work of Dr. Geall, a former UK-based chief researcher at Novartis, who is now an RNA vaccine consultant based in San Diego.
Based on the information provided by Dr. Geall, here are the general steps for creating an mRNA vaccine in the lab:
- Identify the gene sequence of the virus you want to create the vaccine for.
- Design and order custom “oligos,” e.g. lab-generated DNA strands. These are created sequence-by-sequence using an enzymatic process (e.g. solid-phase chemical synthesis) in a method this is analogous to layer-by-layer 3D printing.
- Prepare and assemble (e.g. clone or insert) the desired viral gene sequence into the oligo structure (in this case, we want the gene for the “spike” antigen feature of the virus) to create a linear structure.
- The result is a template structure, known as a plasmid DNA replicon, which is used to make the RNA vaccine copies. (Template plasmid DNA structures are independent of our normal cellular DNA.)
- Produce the RNA vaccines using in vitro transcription (IVT) e.g. a combination of enzymes and the plasmid DNA replicon template (a process known as enzymatic transcription reaction.)
- Purify the results to eliminate by-products, such as any leftover enzymes or DNA fragments, and “cap” the ends of the mRNA strands.
- Sterilize the results by passing it through a 0.2 µm filter.
- Begin testing the results, e.g. the RNA vaccine samples, for efficacy.
So, while we aren’t literally printing vaccines atom-by-atom, we are using an approach that’s reminiscent of 3D printing, particularly in the creation of custom oligos, which lab specialists painstakingly create, gene-by-gene (using enzymes) to create a custom DNA template that will in turn “print out” copies of mRNA vaccines.

Ramping up Mass Production: the Four Key Steps in Manufacturing MRNA Vaccines against Covid-19
Just as it’s a big leap from creating a prototype part using a 3D printer in a lab or a makerspace to going into mass production at a factory, so too is it a massive leap from creating a small batch of mRNA vaccine in the lab to producing millions and millions of vaccine doses at scale.
Let’s look at some of the interesting aspects of scaling up production from small, lab-produced samples to millions of doses.
A disclaimer: we have been keeping up with current and past announcements from both Moderna and Pfizer/BioNTech to stay up to date on their latest manufacturing processes; however, many of their latest processes are not fully documented and/or are proprietary, so some of this information may not reflect the very latest innovations.
Step One: Bioreactor Production
The first step in manufacturing the coronavirus vaccine is to create the active ingredient, e.g. the strands of mRNA.
Here, the process is similar in principle to the methods used in the laboratory above but scaled up into larger bioreactor vessels (kettles) to produce more vaccine product per batch.
What’s remarkable about mRNA factory production is that it takes remarkably less floor space than earlier vaccine production methods, such as those commonly used for flu vaccines that rely on using a single chicken egg per dose. Nor does this process require cultivating any living cell cultures (it just uses enzymes to synthesize the vaccine), which helps speeds up the production process and simplifies raw material in the supply chain. Finally, each dose is incredibly potent, so the actual volume for each dose is very minute – this results in much higher yields per batch, which significantly increases production efficiency.
A promotional video produced by Pfizer indicates that they have split up their bioreactor production into different factories. While Pfizer produces all the template plasmid DNA used worldwide at its Chesterfield production plant outside St. Louis, Missouri, the subsequent DNA transcription process takes place either at their Andover plant outside of Boston (for North American production) or is sent to the BioNTech plant outside of Marburg, Germany (for European production).
Moderna’s US production is located outside of Boston, while their European product is produced in Visp, Switzerland.
Step Two: Initial Downstream Purification
The raw mRNA produced in the bioreactor process is not ready to be used yet.
In this step, it will be processed to remove extra DNA elements left over from the transcription process as well as to remove any enzymes. The mRNA also needs to be “capped” at the ends.
Purification is important because any extraneous elements could trigger an unwelcome immune response from the human immune system – but not the one we are looking for!
Step Three: Additional Purification, LNP (Lipid Nanoparticles) Encapsulation, and Sterilization
The next step is one of the most remarkable ones, the encapsulation of the fragile mRNA into a more durable lipid nanoparticle (LNP), essentially a microscopic droplet of oily fat.
LNPs are one of the enabling technologies for mRNA vaccines. Until they were developed into a reliable, mass-producible technology (we’re currently using 3rd generation LNPs), the whole idea of using RNA as a therapeutic seemed impractical. Sure, you could make some RNA in the lab, but it would soon degrade, rendering it unsuitable for mass production.
The third generation LNP encapsulation is the smallest yet, and that allows it to pass through standard 0.2 µm filters to ensure proper sterilization.
Interestingly Pfizer transports its vaccine components to yet another factory setting for the LNP encapsulation, final purification, and sterilization: product intended for North America is sent to Pfizer’s Kalamazoo, Michigan facility while product intended for European markets is processed at their Puurs, Belgium plant (as well as a new, ex-Novartis plant in Stein, Switzerland).
Moderna’s North American LNP encapsulation processes are understood to be in Massachusetts, and their European LNP encapsulation is handled by Rovi Pharma Industrial Services in Spain.
Step Four: Fill-to Finish Operations and Overcoming Cold Chain Logistics Challenges
The next step in the manufacturing process is the traditional “fill-to-finish” operation, in which vaccine product is filled into glass vials and packaged into boxes for shipment.
Here the challenges had more to do with the unique need to keep mRNA product at extremely cold sub-zero temperatures (to prevent degradation of the fragile mRNA molecules) as well as supply-chain challenges to source millions of tiny glass vials, syringes, etc. during a time when manufacturing production, warehousing, and shipping logistics was so heavily impacted by Coronavirus lockdowns and labor shortages due to ill workers.
Once the product is packaged for shipment, new transportation methods had to be introduced to keep it at sub-freezing temperatures – between -80°C and -60°C (-112°F and -76°F) – requiring logistics companies such as FedEx and UPS to beef up their existing “coldchain” transportation systems.
But despite these challenges, Moderna and Pfizer/BioNTech have been able to successfully deliver millions of doses around the world.
Coming Soon: Using mRNA as a Drug Delivery Platform
So what can we expect next?
Now that the Modern and Pfizer mRNA vaccines have come to market, it will be easier to repeat the process to design updated vaccines to address the slightly different genetic codes of the newer Covid variants.
And this represents a major technological advance over previous vaccines development programs – indeed, what the Pharma industry as a whole is in the process of creating is a therapeutic delivery “platform” – one where all of the technology for manufacturing and delivery can remain the same, the only change needed to target a specific disease is to design and insert the relevant genetic code.
What does this mean in practical terms?
One advantage is speed.
By creating a factory production “platform”, pharma companies will be able to react quickly to new infectious diseases as they emerge – as well offer new therapeutic treatment options for diseases that have proved difficult to cure.
Meanwhile, Moderna plans to use its mRNA “bio-platform” as a basis for developing and delivering new vaccines against the flu, zika, HIV, and cancer.
If the confidence of these drug researchers bears out, we could see a dramatically different pharma landscape in the coming years.

Formaspace Is Your Drug Development Facilities Partner
If you can imagine it, we can build it, here at our factory headquarters in Austin, Texas.
Formaspace builds custom laboratory furniture for commercial and educational lab facilities as well as custom industrial furniture installations for pharma manufacturers around the world.
Contact your Formaspace Design Consultant today to find out how we can work together to make your next project a success.