FDA Approves a New Treatment for Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig’s Disease
In an interview with Neurology Live, Lawrence Steinman MD, Professor of Neurology at Stanford University, describes the grim reality of living with Amyotrophic Lateral Sclerosis (ALS) in just a few words:
“(ALS is) one of the most terrifying diseases we homo sapiens have to face. It’s very different than Alzheimer’s dementia because your thought processes are entirely intact and you just can’t move, except for your eye muscles.”
Given these devastating outcomes, the ALS community welcomed the news that the FDA approved a new drug, AMX0035 (a co-formulation of sodium phenylbutyrate and taurursodiol) in September 2022, making it the third drug after Edaravone (approved in 2017 and marketed as Radicava) and Riluzole (approved in 1995 and marketed as Rilutek) available for treating ALS patients.

ALS Drug Approval Marks Another Controversial Decision by the FDA
Following the FDA’s controversial approval of the Alzheimer’s drug Aduhelm in 2021 – a drug that was ultimately withdrawn from the market due to a lack of willingness by insurance companies (who questioned its efficacy) to pay for it – few expected the agency to step into controversy again so soon.
Indeed, an 18-month-long investigation by the House Oversight and Reform Committee found, among several anomalies, that the FDA coordinated with drugmaker Biogen “inappropriately” during the early stages of the process – adding fuel to the fire for FDA critics claiming the agency is too ‘cozy’ with pharma companies.
But we digress.
The approval process for AMX0035 was also unconventional, but for different reasons.
The Phase 2 study for AMX0035 (dubbed CENTAUR) was relatively small, with only 137 patients participating. The results, published in the New England Journal of Medicine, indicated those receiving the drug had slower functional decline over 24 weeks, but secondary outcomes were not significantly different between the control (placebo) group and those administered AMX0035. The report concluded that “longer and larger trials are necessary to evaluate the efficacy and safety of sodium phenylbutyrate–taurursodiol in persons with ALS.”
In light of the CENTAUR trial results, the FDA briefing documents said the evidence was not “sufficiently independent or persuasive” to warrant approval, leading the FDA’s peripheral and central nervous system drugs advisory committee to vote down the drug 6-4 in March 2022.

The ALS Community Advocated for Approval of AMX0035 despite the Small Phase 2 Study Size and Lack of Clear-Cut Conclusions
After the initial thumbs down from the advisory committee, the drug maker Amylyx submitted additional supporting evidence.
At a second public hearing, it became clear that the ALS community (30,000 Americans are estimated to have ALS) was not ready to give up on any clinical therapy that could help patients survive for even a few additional months – given the reality that people with ALS have a short life expectancy (typically 2 to 5 years after diagnosis), and there is no known cure.
In a press release, the ALS Association recounted the testimony given at the FDA’s public hearings on AMX0035, including by its Board of Trustees chair, Scott Kauffman, who spoke passionately about caring for his son with ALS: “AMX0035 represents a meaningful step forward in progress. (An additional) Ten months is a long time for someone living with ALS.”
In light of the new submissions and public input, the FDA advisory committee reversed itself, voting 7-2 in favor of accelerated approval, which the FDA formally agreed to on Sept 29, 2022.

If the New ALS Drug Does Not Demonstrate Efficacy in Larger Trial Studies, the Drug Maker Amylyx Says it Will Voluntarily Withdraw it from the Market
Given the unexpected FDA approval of the Alzheimer’s drug Aduhelm in 2021 and the ALS AMX0035, what conclusions can we draw about the future of FDA drug approvals?
We’re reminded of the keynote address that the former head of the FDA, Stephen M. Hahn M.D., gave at the 2021 Texas Bioscience Conference in which Hahn expressed his belief that the FDA needs to be nimbler in its approach to drug approval. Instead of relying on a series of clinical trials that could take years, Hahn spoke in favor of an “expedited approval” process that allows urgently needed drugs to be approved earlier in the process, as long as the approval is subject to revocation if subsequent trials and results in the field contradict the early positive results.
In the case of AMX0035, no safety concerns had emerged during the Phase 2 CENTAUR Trial, and the director of the FDA Office of Neuroscience, Billy Dunn, reached what looks like a “gentleman’s agreement” with Amylyx to voluntarily withdraw the drug if the upcoming larger Phase 3 trial scheduled to complete in late 2023 or 2024 failed to demonstrate its efficacy.
What is the Cause of ALS Disease and How Does the New ALS Drug from Amylyx Help Patients?
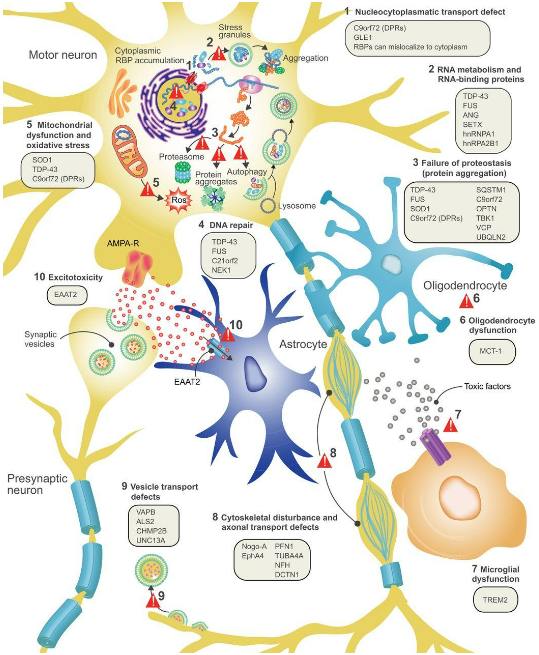
The motor nervous system that allows our brain to control our muscles is complex, and the disease mechanism (or mechanisms) that cause amyotrophic lateral sclerosis is not fully understood. As shown in the diagram above, there are at least 10 processes that could be at fault, including failures in RNA and DNA repair, mitochondrial dysfunction, inflammation of the neurons, defective axonal or vesicular transport, or a build-up of extracellular glutamate, leading to over stimulation of glutamate receptors and excessive calcium.
Whatever the cause(s) of the disease, the result is the same.
In the fairly blunt words of Lawrence Steinman MD, Professor of Neurology at Stanford University (whom we quoted at the beginning):
“…in ALS, the motor neuron is sending out a danger signal, the motor neuron is expressing itself chemically, saying “I’m sick.” And then guess what happens, the big “eaters”, the macrophages, and their counterparts in the nervous systems, the microglia, destroy it (the motor neuron). And once your motor neuron is destroyed, you have no muscle power, your muscles atrophy and that’s the story.”
So, if we don’t yet know what causes ALS (Dr. Steinman thinks it’s related to a protein called Integrin alpha 5), then how exactly does AMX0035 help ALS patients?
The answer is we don’t.
Per the FDA’s prescribing information, “the mechanism by which RELYVRIO (Editor note: the brand name of AMX0035) exerts its therapeutic effects in patients with ALS is unknown.”

Amylyx Announced their New ALS Drug, now called Relyvrio, Will Cost $158,000 for the First Year of Treatment
What we do know is the price.
This new ALS drug, brought to market as Relyvrio, is expected to cost $158,000 for the first year of treatment, rising to around $163,000 for the second year.
The high cost is in line with another ALS drug, Edaravone (marketed as Radicava), which costs $165,300 annually. However, due to its high cost, Edaravone is rarely approved for reimbursement by insurance agents – and insurance industry experts predict that Relyvrio will also meet fierce resistance among insurers.
Instead, most ALS patients turn to Riluzole (marketed as Rilutek) now that its patent (issued in 1995) has expired, creating a market for generic versions that are available for as low as $1,150 a year.
Enterprising ALS patients have also tried to replicate the ingredients in Relyvrio, which is a combination of sodium phenylbutyrate and taurursodiol. However, sodium phenylbutyrate is still quite expensive (estimated to cost $60,000 annually) and, while taurursodiol is available as a supplement, there are concerns about its purity and efficacy when purchased over the counter.
Formaspace is your Partner for Pharma Laboratory Research
If you can imagine it, we can build it, here at our Austin, Texas, factory headquarters.
Talk to your Formaspace Design Consultant today about how we can work together to make your next laboratory design project or remodel a success.