The Autoimmune Disease Lupus and its Effects on the Body
The CDC estimates that circa 200,000 adult Americans suffer from lupus, officially known as Systemic Lupus Erythematosus (SLE). The disease disproportionately affects women (in 90% of cases), with Black and Latina women at the highest risk – 1.5 to 3 times more likely to develop lupus than White women.
SLE is understood to be an autoimmune condition in which the body’s immune system mistakenly attacks healthy tissue, often in recurring episodes of varying degrees of intensity and duration.
According to rheumatologists who study and treat immune system diseases of the bones, joints, and connective tissues and muscles, the most common symptoms of SLE include fatigue, painful joints, skin rashes, swollen lymph nodes, mouth ulcers, and hair loss. SLE is also associated with other conditions, including serositis (inflammation of the linings of the heart and lungs), kidney disease (particularly in male patients), and peripheral neuropathy.
What is the CAR T-Cell Mechanism, and How Can it Treat Autoimmune Diseases such as Lupus?
While we can’t currently pinpoint why some people begin to suffer from lupus yet others do not, researchers have developed a clearer understanding of the underlying disease mechanism.

In the case of lupus, the body’s immune system receives a cellular signal to fight against suspected pathogens. However, this is a “false flag” attack – instead of attacking true pathogens, the body’s immune system is mistakenly sending in B cells to attack its own natural proteins. Worse, these “autoreactive” B cells erroneously create antibodies to fight these proteins, essentially creating a vicious circle of cause and effect – the body attacks itself, creating inflammation, which aggravates the condition, causing the immune system to double down its attack.
Available therapies to address SLE symptoms, such as administering steroids and NSAIDs to reduce inflammation, do not address the underlying disease and can cause additional problems when used over the long term.
To address the root cause of SLE, e.g. break the cycle of autoimmune attacks, researchers looked for ways to interrupt or even remove B cells entirely.
One approach is to use monoclonal antibody therapies to target the surface markers on dysfunctional B cells and destroy them. Two such therapies include the anti-CD20 antibody Rituximab (available under the brand name Rituxan) and Obinutuzumab (marketed as Gazyva). Frustratingly, however, clinical trials of drugs such as Rituximab did not lead to full remission of the disease, perhaps due to the transient or incomplete effect of B cell depletion.
So researchers began to wonder: “could the body’s immune system be trained to manage the errant B cells directly?”

To address this question, SLE researchers in the 2010s began to look at the success cancer researcher were having with patient-specific CAR T-cell-based therapies as a potential option for treating lupus.
In this pioneering approach to treating cancer, researchers draw blood from cancer patients to extract disease-fighting T-Cells, then genetically modify them to train their chimeric antigen receptors (CARs) to identify and destroy cancer cells before transfusing them back into the patient’s bloodstream.
Mouse Model Research Points the Way for Treating Systemic Lupus Erythematosus (SLE) with CAR T-Cell Therapy

Researchers led by Dr. Rita Kansal at the University of Tennessee Health Science Center in Memphis began evaluating the idea of using CAR T-cell therapy for lupus by creating two different Lupus disease models in mice (NZB/W and MRL-lpr) using a combination of healthy and autoimmune compromised mice.
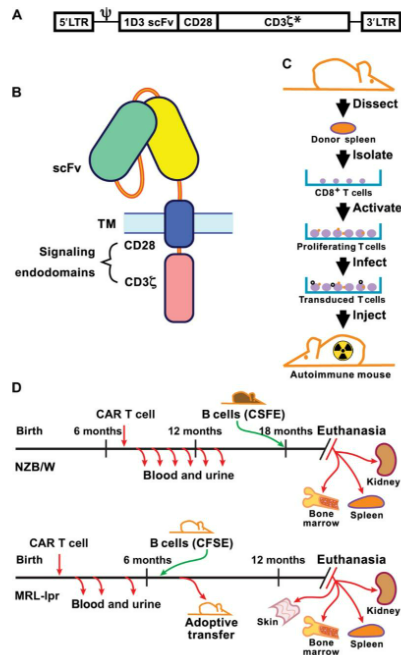
As described in a landmark paper published in 2019, the researchers were able to extract CD8+ T-cells from the spleens of healthy mice and transduce (e.g. genetically modify) the cells to express CD19-targeted chimeric antigen receptors (CARs).
These modified T-cells were injected into autoimmune mice, where they successfully depleted the CD19+ B cells for up to a year, breaking the cycle of autoimmune disease proliferation.
The mice receiving CAR T-cell therapies (NZB/W and MRL-lpr) lived significantly longer lives post-treatment, with the majority living an average of 18 months (NMZ/W-treated mice) or 14 months (MRL-lpr mice). This compares favorably to the untreated NZB/W and MRL-lpr control mice which survived a mean average of 200 days or less.
CAR T-Cell Therapy Study in Experimental Human Trials Demonstrates Significant Signs of Remission in 6 Lupus Patients
One of the pioneering researchers in developing CAR T-Cell therapy protocols for treating lupus in human subjects is Georg Schett, MD, and his team at the German Center for Immunotherapy at the Friedrich Alexander University in Erlangen, Germany.
In a letter published in the New England Journal of Medicine in 2019, Dr. Schett’s team announced that they had used CD19 CAR T-cells to treat a 20-year-old woman with an advanced case of lupus that had failed to respond to numerous treatments, including monoclonal antibody therapies (Belimumab and Rituximab) targeting B cells.
According to Dr. Schett’s team, the woman receiving the experimental CD19 CAR T-cell therapy appeared to respond as hoped. The CAR T-cells circulating in the bloodstream increased rapidly during the first nine days, followed by the “complete and sustained depletion of circulating B cells. This corresponded to an observable remission of the patient’s clinical symptoms of lupus without any negative side effects attributable to the treatment.

Encouraged by these results, the researchers expanded the experimental human trial to evaluate another five patients (four women and one man) who have lupus. The results of this second round of human trials were recently published in Nature.
As before, T-cells were drawn from each patient and transduced using a lentiviral anti-CD19 CAR vector, then each unique formulation was reinjected back into each individual patient.
As happened during the first human trial, the number of CAR T-cells rose rapidly, followed by a deep depletion of B cells and a marked improvement in clinical symptoms and results from standardized clinical tests.
After three months, the patients entered remission according to the DORIS evaluation test criteria.
At about 8 months, monitoring tests indicated a return of B cells in the blood, but they did not exhibit dangerous autoimmune behavior. To some researchers, this indicates that the newly generated B cells emerging from the bone marrow were unaffected by the original onset of lupus.
Speaking to Healio magazine, Dr. Geog Shett said “We were excited from the beginning, and we continue to be thrilled by the response we observed. Of course, we are actually very happy to see that the approach is feasible in lupus… before the first patient, we did not know what would happen if you expanded T cells from an autoimmune patient and reinjected them. We thought it might cause problems like inflammation, but that was not the case.”
Dr. Shett cautions that it’s too early to say the Lupus patients are cured, but the results are encouraging. “It’s pretty touching that you change the life of a young person,” Schett told STAT.
Overwhelming Demand for Access to CAR T-Cell Therapies
Given the potential that CAR T-cell therapy could offer Systemic Lupus Erythematosus (SLE) patients long periods of remission – or potentially even a cure – it’s not surprising that patients are seeking ways to gain access to this revolutionary experimental treatment.
This mirrors the problem of high demand and limited supply that oncologists have encountered trying to source experimental “compassionate use” CAR T-cell-based cancer treatments for their patients.
Currently, any form of CAR T-cell therapy is expensive – treatment costs are estimated at $400,000 or more per patient.
Because the therapy is personalized for each patient, specialty clinical laboratories are finding it difficult to scale up production despite increasing demand.
Hopefully, the supply issues can be addressed quickly, as CAR T-cell therapy may prove a valuable treatment for other autoimmune conditions where B Cells that have gone awry are thought to be responsible, including systemic sclerosis, multiple sclerosis, Chron’s disease, plaque psoriasis, and rheumatoid arthritis.
Formaspace is Your Laboratory Research Partner
If you can imagine it, we can build it, here at our factory headquarters in Austin, Texas.
Talk to your Formaspace Design Consultant today to find out how we can work together to make your next laboratory build project or remodel a success.