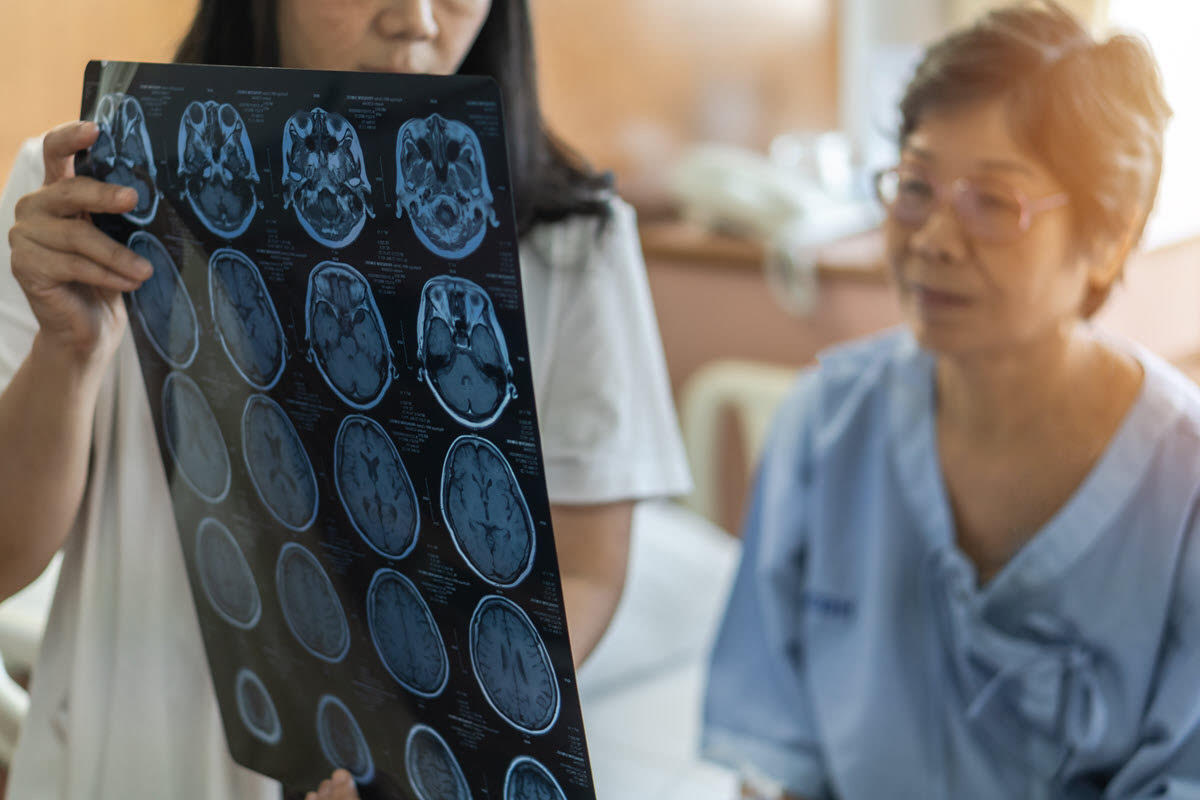
The Projected Number of Future Dementia and Alzheimer’s Disease Cases is Staggering
A new international study published in The Lancet Public Health estimates that dementia cases worldwide will increase by a factor of 3 between 2019 and 2050, with Africa and the Middle East expected to have the highest increases. US dementia cases will double during this time period, jumping from around 5 million plus cases to around 11 million by 2050.
Among the different forms of dementia, Alzheimer’s disease is the most common; the WHO estimates that it accounts for between 60 and 70% of dementia cases.
These are devastating numbers.
Unless something can be done quickly, millions of individuals (and their family members who will have to look after them) will suffer tremendously from the burden of an Alzheimer’s diagnosis. And the impact will also be felt throughout the public healthcare system due to rising costs for disease management and providing long-term care.
Given the urgency to avert this looking public health crisis, are we on the verge of discovering new diagnostic tests and clinical therapies for Alzheimer’s disease?
We take a look at the current advances in medical laboratory research.

Is there is a Single Cause of Alzheimer’s Disease?
The billion-dollar question is whether Alzheimer’s is caused by a single disease mechanism or by multiple factors.
Many hope it’s the former and that they can repeat the success of Australian doctors J. Robin Warren and Barry Marshall, who discovered in 1983 that peptic ulcer disease (PUD) was primarily caused by a bacterium (Helicobacter pylori). This discovery, for which they were awarded the Nobel Prize in 2005, upended decades of conventional clinical treatment therapies to treat peptic ulcers, including administering strong anti-acids, drinking milkshakes (which ironically helped feed the bacterial infections), and undergoing stomach surgeries to remove diseased tissue.
We don’t hear a lot about peptic ulcers today, as most of them can be cleared up by taking strong courses of antibiotics.
Could a similar “silver bullet” discovery help us reverse the progress of Alzheimer’s disease? Or will it prove to be a much more stubborn opponent, a tricky combo act comprised of multiple diseases working together to disable our cognitive functions?
Alzheimer’s Disease Correlation and Causation, Trying to Determine Which is Which
Hope for an Alzheimer’s cure has been raised many times.
We often read about what appear to be statistically significant correlations between Alzheimer’s diagnoses and different demographic cohorts.
For example, people with higher education attainment are, statistically speaking, less likely (on average) to be diagnosed with dementia, including Alzheimer’s.
This is useful information for public health professionals, and indeed, the worldwide dementia study mentioned up top, incorporates education as a factor when making projections about how many dementia cases are expected in 2050.
But, as you most likely learned in a statistics course, correlation is not causation.
It’s quite reasonable to assume educated people have some behavior that reduces their dementia risk; however, it’s not scientifically sound (at least to our knowledge) to assume that taking a college class later in life, studying a new language, or playing “brain teaser” puzzles will help avert dementia or Alzheimer’s down the line. Maybe it will, maybe it won’t. We just don’t have proof that the lack of high-level mental activity is causation of the disease.
Alzheimer’s Disease Causation and Risk Factor Theories over the Years
This correlation/causation dilemma has been an ongoing challenge in trying to bring Alzheimer’s to heel. Over the years, there have been many tantalizing studies that correlate the onset of the disease with other chronic health comorbidities such as obesity, smoking, and high blood sugar (diabetes).
Different disease mechanism hypotheses have emerged over the years to explain how and why patients develop Alzheimer’s disease; here are some of the most prominent ones:
· Type III Diabetes Theory
Scientists have speculated that Alzheimer’s actually could be a third type of diabetes. This “Type III” diabetes theory suggests that overproduction of insulin is involved in: the formation of amyloid–β (beta) plaques in the brain (which may inhibit memory) and the phosphorylation of tau, which helps product neurofibrillary tangles (which would disrupt memory cells) – two of the leading suspects in Alzheimer’s disease.
· Autoimmune Disease Hypothesis
Because of the strong association between inflammation and Alzheimer’s, researchers have sought to determine if the disease is caused by some form of auto-immune disease in the brain. According to this theory, microglia cells in the brain, which are responsible for the health of neurons and synapses, could be responsible for overreacting to the presence of an infection or an unexpected molecule, such as amyloid-β. In doing so, the hypothesis speculates that the cleanup process doesn’t work properly, leading to a dangerous “friendly fire” buildup of microglia complexes (inflammasomes) that don’t disperse as would be expected, leading to further inflammation, long-term neurodegeneration, and ultimately memory loss and cognitive decline.
· Dangerous Proteins: Transmissible Prions Hypothesis
Our understanding of proteins changed dramatically in 1982 when Stanley Prusiner, now of UCSF, first proposed the idea that proteins, once thought to be incapable of replicating, could cause infectious diseases. Dubbed “prions” from the words ‘proteinaceous’ and ‘infectious,’ prions are associated with progressive neurological disorders such as Classic Creutzfeldt-Jakob Disease (aka Classic CJD) and the cattle-born related disease Bovine Spongiform Encephalopathy (BSE), commonly known as Mad Cow Disease. Could Alzheimer’s also be transmitted by a prion, perhaps by a prion form of an amyloid?
· Gum Disease
The presence of bacteria in the brains of Alzheimer’s patients has led to another novel theory. Could gum disease caused by bacteria in the mouth (such as Porphyromonas gingivalis) somehow cross the blood-brain barrier and cause brain plaques associated with Alzheimer’s? According to this theory, the brain rushes to protect itself from bacterial infections due to periodontal disease – but in doing so damages healthy brain tissue by creating a buildup of amyloid plaque deposits which degrade memory function.
· Nutrition Deficiencies
More nutritious diets, such as the low-fat Mediterranean and MIND diets, are associated with a reduced incidence of Alzheimer’s disease. But this may be another case of correlation rather than causation. Those that follow these diets, which emphasize eating less red meat in favor of a wide variety of foods, including vegetables, seafood, and healthy oils (such as olive oil), may indeed help reduce their risk for cognitive decline, but it’s also possible they represent more health-conscious statistical cohort. If so, then there must be other causation factors that determine who will get Alzheimer’s.
· POCD from Anesthesia during Surgery
Could administering general anesthesia during surgical operations lead to long-term cognitive problems, including the onset of Alzheimer’s? This hypothesis arose from concerns about patients who experience Postoperative Cognitive Dysfunction (POCD), e.g., cognitive problems after surgery that persist long after anesthetics effects have worn off. Scientists are researching POCD to identify whether it is associated with inflammation of the central nervous system (e.g., neuroinflammation) and if it also contributes to the development of Alzheimer’s disease.
· Sleep Disorders
Could sleep disorders and disruptions to our internal 24-hour circadian clock contribute to Alzheimer’s? This theory surmises that good sleep helps give processes in the brain time to repair damage to memory cells, for example, by allowing microglia immune cells to remove unwanted accumulations of amyloid beta protein plaques (which are known to be present in patients with advanced cases of Alzheimer’s). If this association with sleep is true, then we need to redouble our efforts to improve “sleep hygiene” to stave off the onset of Alzheimer’s.

The Big Data Approach to Resolving Correlation and Causation at the Genetic Level
You might be asking yourself, “couldn’t a ‘big data’ approach to genetic analysis help resolve the debate about the correlation and causation of Alzheimer’s?”
We are not there yet, but major progress has been made in genetic research to identify the specific genes associated with an increased risk of Alzheimer’s disease.
For example, researchers at the UK Dementia Research Institute recently published a paper in Nature Genetics that identifies 75 genes, including 42 genes not previously identified, that appear to be associated with Alzheimer’s progression.
It is hoped that identifying these genes will not only help scientists understand the pathophysiological processes that lead to the progression of Alzheimer’s, they will also help us identify useful biomarkers to help clinicians make definitive Alzheimer’s diagnoses as well as help drug developers design specific therapeutic targets for preventing, treating, or reversing the disease.
Major Focus Areas in Current Alzheimer’s Disease Research
As we’ve outlined above, there are multiple theories seeking to explain the development of Alzheimer’s disease.
But what are the main areas of focus right now?
Broadly speaking, most researchers are looking at these three different disease mechanism theories:
· Amyloid-β Protein Research
Researchers first published the hypothesis that amyloid protein plaques were associated with Alzheimer’s disease back in 1984. Additional research in the early 1990s continued to focus on this theory, seeking to understand the role that amyloid-β peptides (which form molecular clumps in the brains of Alzheimer’s patients) play in developing the disease.
Scientists began to speculate about the possibilities of developing specific clinical therapies that would target these amyloids.
But time and time again, researchers have created drugs that target Amyloid-β plaque buildup, only to meet disappointment when they failed to perform in clinical trials, including a major effort by Pfizer and one from Lilly.
Fast forward to the year 2021, when the FDA approved the first clinical therapy that targets the accumulation of soluble and insoluble forms of amyloid-β in the human brain.
The new drug (aducanaumab) is an immunoglobulin gamma 1 (IgG1) monoclonal antibody developed by Biogen (marketed under the trade name Aduhelm).
Despite the initial excitement, the reception of the new drug has been tempered by controversies.
It started with the approval process, overseen by then the head of the FDA, Dr. Steven Hahn, which became highly contentious, with disputes spilling out into the public arena. Several prominent scientists resigned from the FDA committee in protest during the review hearings due to concerns over Aduhelm’s limited efficacy, and to date, most insurers (including Medicare) have taken a ‘wait and see’ approach before adding it to their prescription formularies.
(See our coverage of the recent Texas Life Science Forum, where Dr. Hahn gave the keynote address.)
Others have raised concerns that the controversial approval of Aduhelm could negatively impact other Alzheimer drug programs in the pipeline (including one that targets an amyloid precursor protein, or APP), as well as those in development for other neurodegenerative diseases, such as Huntington’s and Parkinson’s.
In response to reimbursement limits, particularly by Medicare, Biogen recently pulled back its efforts to market Aduhelm.
· Cholinergic Hypothesis
Our knowledge of how the brain manages its stored memory has evolved a great deal since the Amyloid-β protein causation theory for Alzheimer’s was first proposed in 1984.
For example, recent research investigating neuronal ensembles that hold individual memories (known as “memory engrams”) has found evidence that specific memories are actually distributed across different parts of the brain – a surprising result.
Thus, interfering with the neurotransmitters that communicate across different parts of the brain could be a cause of Alzheimer’s. To explain how this process could get disrupted in Alzheimer’s patients, scientists have developed a “cholinergic system” hypothesis. In this hypothesis, something interferes with the Cholinergic synapses in the brain (e.g. the chemical synapses that use acetylcholine molecules as neurotransmitters), resulting in a breakdown in the communication channels needed to manage memories in the brain (and thus explaining the memory degeneration experienced by Alzheimer’s patients.)
Administering Cholinesterase inhibitors, which have already seen some limited success in treating Alzheimer’s’ disease dementia, might be the solution to preventing or even reversing the disease. Time will tell.

· Tau Protein triggering Neurofibrillary Tangles
We’ve discussed the observable buildup of Amyloid-β protein plaques in the brains of patients with late-stage Alzheimer’s.
Along with these plaques, researchers have also long been concerned about the presence of neurofibrillary tangles in Alzheimer’s patients as well; these appear to be caused by the presence of a protein called tau.
Many studies have been conducted to try to understand whether the observable presence of tau proteins (like their Amyloid-β protein brethren) is a cause or a consequence of Alzheimer’s. The jury is still out. Clinical trials are also underway to determine the efficacy of therapies that target the tau proteins that seem to create these neurofibrillary tangles in the brain.
More recently, research coming out of the lab at UC Riverside has identified that there are actually both left- and right-handed versions (or “isomers”) of tau proteins. Most amino acids in living organisms are left-handed, so researchers are very interested to find out if these right-handed versions of tau proteins could be dangerous to neurotransmitters, and if so, how the body can be encouraged to “purge” them (a process known as autophagy) before they can damage the brain’s memory functions.
In conclusion, we’re getting closer than ever to finding the cause of Alzheimer’s, and hopefully, new clinical tools that can help provide definitive diagnoses, as well as clinical therapies that can prevent, treat, or even reverse the disease.

Formaspace is Your Partner for Biotechnology Research
If you can imagine it, we can build it, here at our factory headquarters in Austin, Texas.
Why not contact your Formaspace Design Consultant today and find out how we can work together to make your next laboratory construction project or remodel a resounding success.