Is HBO’s Series “The Last of Us” Pure Entertainment or a Dystopian Prediction for the Future?
Coming off the tail end of the Covid pandemic (knock on wood), the sudden popularity of HBO’s apocalyptic horror series “The Last of Us” may come as a surprise for those of us looking for a light entertainment plot that does NOT revolve around a pandemic – especially one featuring out-of-control zombies borne from dangerous, delirious fungal infections in the brain.

But let’s cast aside the commentary on whether the pandemic horror movie genre (which includes the breakout film “13 Monkeys” from a generation ago) is entertaining or not and instead take a look at the underlying scientific research on the parasitic fungus (Ophiocordyceps unilateralis) that drives the zombie plot of “The Last of Us.”
We can start with two basic questions:
Does Ophiocordyceps unilateralis (also abbreviated as cordyceps) exist, and can it create zombies?
The answer is yes, Ophiocordyceps does exist in real life, and it does create zombies – in some insects such as ants and spiders.
Can fungal infections affect human brains?
The answer is also yes, just not Ophiocordyceps fungus.
Indeed, there have been some dangerous, active outbreaks of fungal infections affecting the brain that has been spreading across the US for decades.
· Coccidioides, the cause of San Joaquin Valley Fever
One is the fungal family Coccidioides, which causes Coccidioidomycosis – perhaps better known as San Joaquin Valley Fever or “desert rheumatism.” This disease is primarily found in southwestern farming communities and is spread by disturbing the soil and breathing in mold spores. In severe cases, it can spread to the lungs (presenting as pneumonia) and into the brain.
· Cryptococcus neoformans, the cause of Cryptococcal Meningitis –
a major risk for HIV patients
Another fungal infection that can affect human brains is Cryptococcus neoformans, a fungus that infects an estimated 150,000 people worldwide each year – resulting in over 110,000 deaths annually from Cryptococcal Meningitis. Like many fungal diseases, Cryptococcus is naturally present in our bodies, but under certain circumstances, it can grow out of control – typically in immunocompromised individuals such as those with uncontrolled HIV disease.

Fungal Diseases are on the Rise, and there are Several Theories Why
So how worried should we be?
A bit worried.
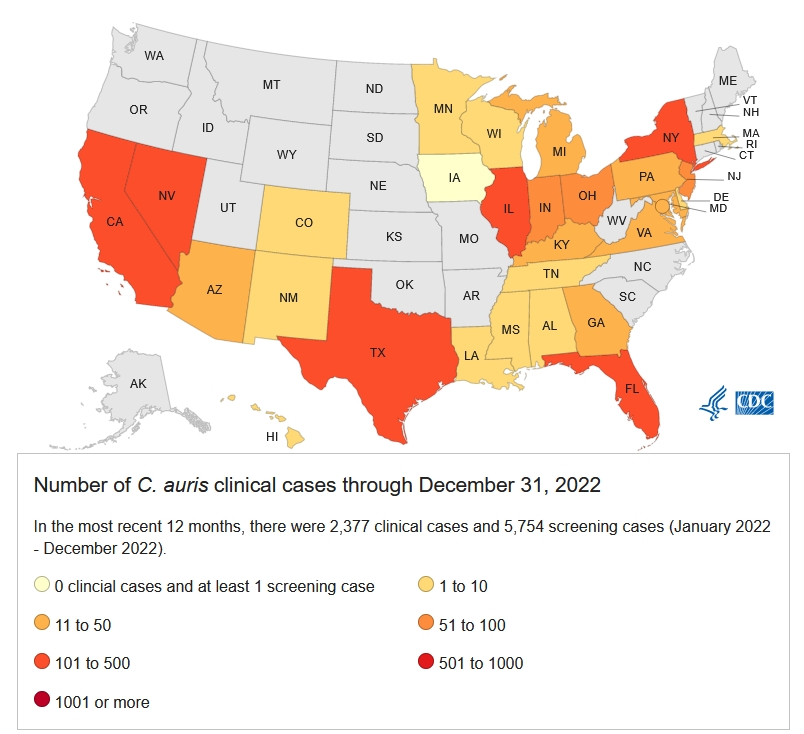
As you are no doubt aware, there is currently an outbreak of drug-resistant Candida auris fungal infections spreading through many US healthcare facilities.
But before we touch on that outbreak (as well as other specific fungal outbreaks), we should as the question why do fungal outbreaks seem to be on the rise?
Fungal disease experts believe that at least two factors are at play.
· Rising Temperatures are Affecting Fungal Reproduction
The first is increased temperatures due to climate change. This past January, researchers at Duke University published a paper on how increasing temperatures affect Cryptococcus reproduction and found some interesting effects. In studying Cryptococcus DNA sequences, they found that when the temperature of the fungal environment was raised from 86 F to 98.6 F, there was an unexpected five-fold increase in certain genomic sequences “jumping” from one part of the fungal DNA to another. Duke researchers speculated that these strange genetic mutations could allow the fungus to adapt to higher heat conditions.

· The Widespread Use of Antifungals in Agriculture is Contributing to Drug-Resistant Fungal Infections in People
The second potential reason that hard-to-treat fungal infections appear to be on the rise is the widespread use of antifungals in agriculture. Each year, a large percentage of our key crop yields are lost to fungal infections, including:
- Up to 40% of strawberries due to gray mold (Botrytis cinerea)
- Around 15% of wheat and barley due to grass mildew (Blumeria graminis)
- Up to 50-70% loss of wheat due to Septoria tritici blotch or rust (Mycosphaerella graminicola / Puccinia spp.)
- Over 80% of some tropical fruits due to Anthracnose spots and blights (Colletotrichum)
- Up to 25% of tomatoes due to Tomato leaf mold (Cladosporium fulvum)
- Up to 60% of potatoes in storage due to dry rot (Fusarium)
- Up to 60-70% of cereals due to Fusarium head and ear blight (Fusarium graminearum)
- Up to 35% of rice due to Rice blast (Magnaporthe oryzae)
- Up to 70% of soybeans due to Rust (Phakopsora pachyrhizi)
- Up to 20% of corn due to Corn smut (Ustilago maydis)
In response, the scientists have developed six main classes of antifungal drugs:
- Methyl benzimidazole carbamate (inhibits microtubule assembly)
- Succinate dehydrogenase inhibitor (inhibits fungal respiration by binding to mitochondria)
- Anilinopyrimidine (inhibits methionine synthesis and hydrolytic enzymes)
- Qo inhibitor (inhibits fungal energy production by binding to the Qo site)
- Morpholine (inhibits ergosterol synthesis)
- Azole (inhibits 14α-demethylase to suppress ergosterol synthesis)
However, scientists are also uncovering the potential risks that the increased use of antifungals in agriculture has on human health.
Researchers at the University of Georgia studied Aspergillus fumigatus, the fungus that causes aspergillosis (which we will discuss below in more detail), and identified a potential causal link between compounds used to fight fungal diseases in plants and the development of resistance to antifungal medications used to treat people. The new research is published in G3 – Genes, Genomes, Genetics – an Oxford Academic journal.

In a paper published this month in Nature Reviews Microbiology, a team of UK and international-based researchers have called for more awareness of the need to balance the relationship between fungus in the environment and human health, an approach known as “One Health.”
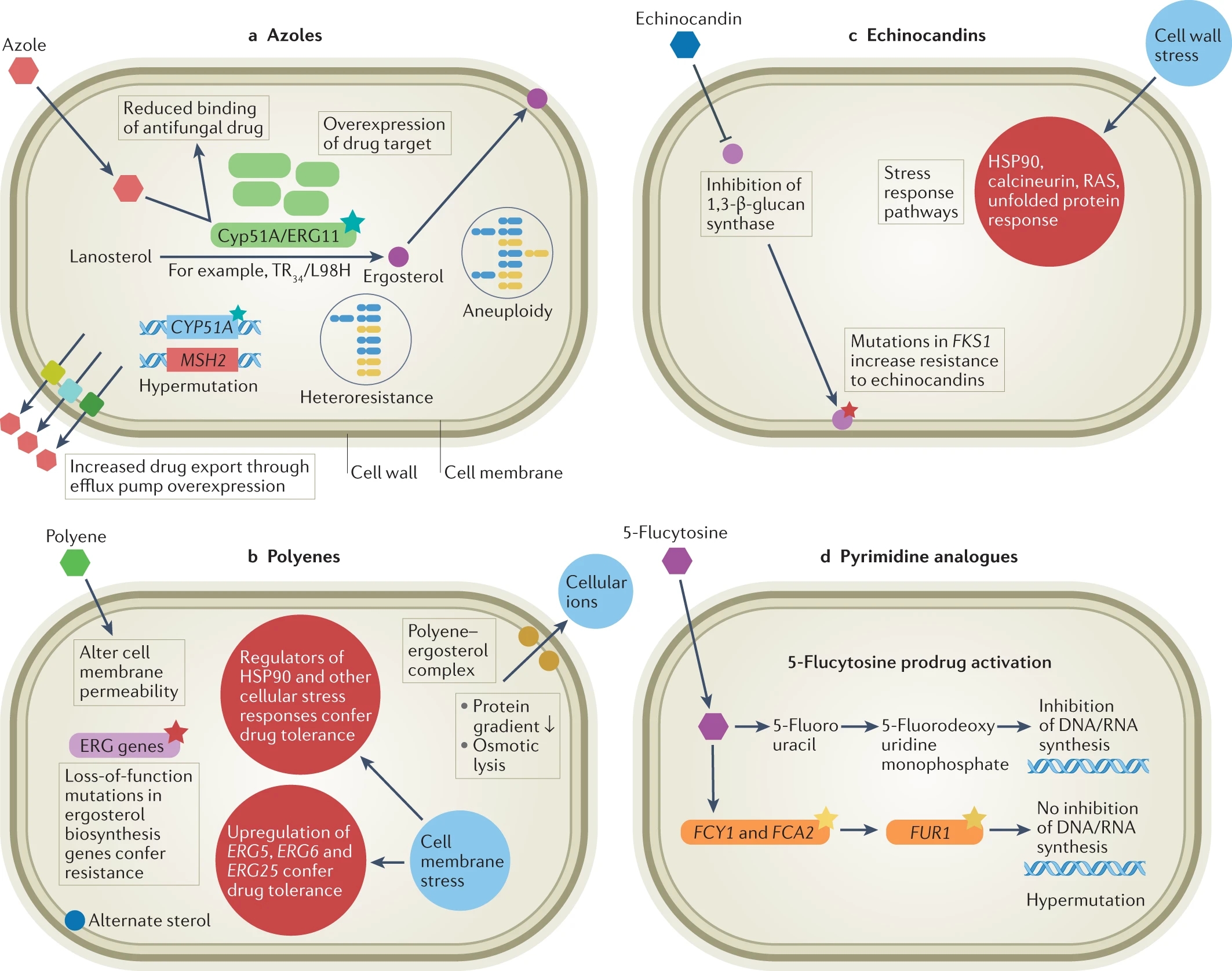
The researchers point out that antifungal drug resistance is an evolutionary response to the selective challenges posed by the drugs we seek to use to control fungal outbreaks – not unlike the issue of antibiotic overuse/misuse leading to the evolutionary development of drug-resistant bacteria.
As Deadly Fungal Infections Rise, the World Health Organization (WHO) Identifies the Most Potentially Dangerous Fungal Outbreaks
Over 75,000 Americans are hospitalized for fungal infections each year, according to the CDC. Deaths from these fungal infections are also on the rise.
In 1969, the CDC reported only 450 deaths in the US due to fungal infections; by 2021, that figure had risen to 7,000 deaths – which may be an undercount due to the lack of widespread testing. Worldwide deaths may be much worse, with some estimating over 1.6 fatalities a year due to fungal infections.
The World Health Organization (WHO) has raised the alarm, calling out 19 species of fungus that require an urgent response from public health authorities and drug makers. Among the most urgent fungal pathogens are Coccidioides and Cryptococcus neoformans which we discussed above.
The other fungal pathogens on the list for which the WHO has raised its highest alarm include:
· Aspergillus fumigatus, the cause of Aspergillosis lung and sinus infections
Infection is caused by inhaling the spores of this common fungus. Normally this does not cause result in a dangerous health condition; however, the fungus can establish itself in the lungs or sinuses or on the skin, and in severe cases, it can spread to affect the brain. Cases of Aspergillus fumigatus infections requiring hospitalization are on the rise.
· Histoplasma capsulatum, the cause of Histoplasmosis, due to ingesting particulates from bat or bird droppings
Histoplasma capsulatum is most commonly spread during demolition or environmental cleanup projects which disturb bat or bird droppings, which release fungal spores into the air. Most common in the Mississippi and Ohio river valleys, Histoplasmosis disease can be mild (and often undetectable) or particularly severe in immunocompromised patients who may experience lung infections that can spread to the brain and spinal cord.
· Candida albicans, a cause of Candidiasis, a human yeast infection
Candida albicans fungus is present on the surface of our skin or mucous orifices. In normal conditions, it does not cause an issue, but it can cause disease when it grows rapidly, potentially infecting the bloodstream or vital organs such as the kidney, heart, and brain.
· Candida auris, a drug-resistant cause of Candidiasis, a human yeast infection
Candida auris is a subspecies of Candida that was discovered relatively recently in Japan back in 2009. In July 2021, the CDC published a worrying note from the field that Candida auris infections circulating in Texas and Washington DC healthcare facilities were resistant to Pan- and Echinocandin-based antifungal treatments. (To give you an idea of the scale of the problem, about 1.5% of cases appear to be resistant to Echinocandin-based treatments.)

Since that time, the fungal pathogen has continued to spread, primarily in healthcare settings, posing a risk for seriously ill or immunocompromised patients. The mortality rate of patients hospitalized with a Candida auris infection can be as high as 60%, although many of these patients are often ill with other serious co-morbidities.
Healthcare providers who detect a Candida auris that is Pan- or Echinocandin-drug resistant can turn to combinations of one or more of the three other commonly prescribed antifungal drug therapies: azoles, polyenes, and echinocandins.
In some cases, a hybrid therapy can knock down fungal infections, but healthcare providers are concerned about the emergence of new Candida auris variants that appear to be resistant to all of the commonly available drug therapies.
In these cases, healthcare providers are turning to extraordinary cleaning procedures as the best way to defeat the spread of Candida auris.
Potential New Antifungal Treatments Now in Clinical Trials
The spread of fungal infections is now on the radar of the American public, thanks to increased awareness of incidents of fungal outbreaks beyond Candida auris, ranging from the recent death of a person due to blastomycosis (caused by ingesting spores of the fungus Blastomyces that lives in decomposing wood and soils) in an area surrounding papermills in Michigan to the spread of an invasive “whiskey fungus” engulfing Lincoln County in Tennessee near the famous Jack Daniels whiskey distillery.
Yet, despite these developments, the WHO says that fungal infections receive less than 1.5% of research funding slated for infectious diseases – and it’s been 20 years since a new class of antifungal drugs has come to market.
Fortunately, there are some promising new antifungal clinical therapies in the drug development pipeline, including:
- fosmanogepix by Pfizer, which targets Aspergillus and Candida
- ibrexafungerp by Scynexis Inc., which also targets Aspergillus and Candida
- olorofim by F2G Ltd., which targets Aspergillus and other rare molds
- rezafungin by Cidara Therapeutics Inc., which targets Candida

We’ll be tracking the development of these antifungal drugs with great interest as they undergo clinical trial testing.
Hopefully, one or more of these new drugs will help prevent us from losing the fight against untreatable fungal infections.
Formaspace is Your Laboratory Research Partner
If you can imagine it, we can build it, here at our Formaspace factory headquarters in Austin, Texas.
Talk to your Formaspace Design Consultant today. Find out how we can make your next lab project build-out or remodel a complete success.