For the First Time, the FDA Approves Gene Editing Therapies for a Chronic Disease
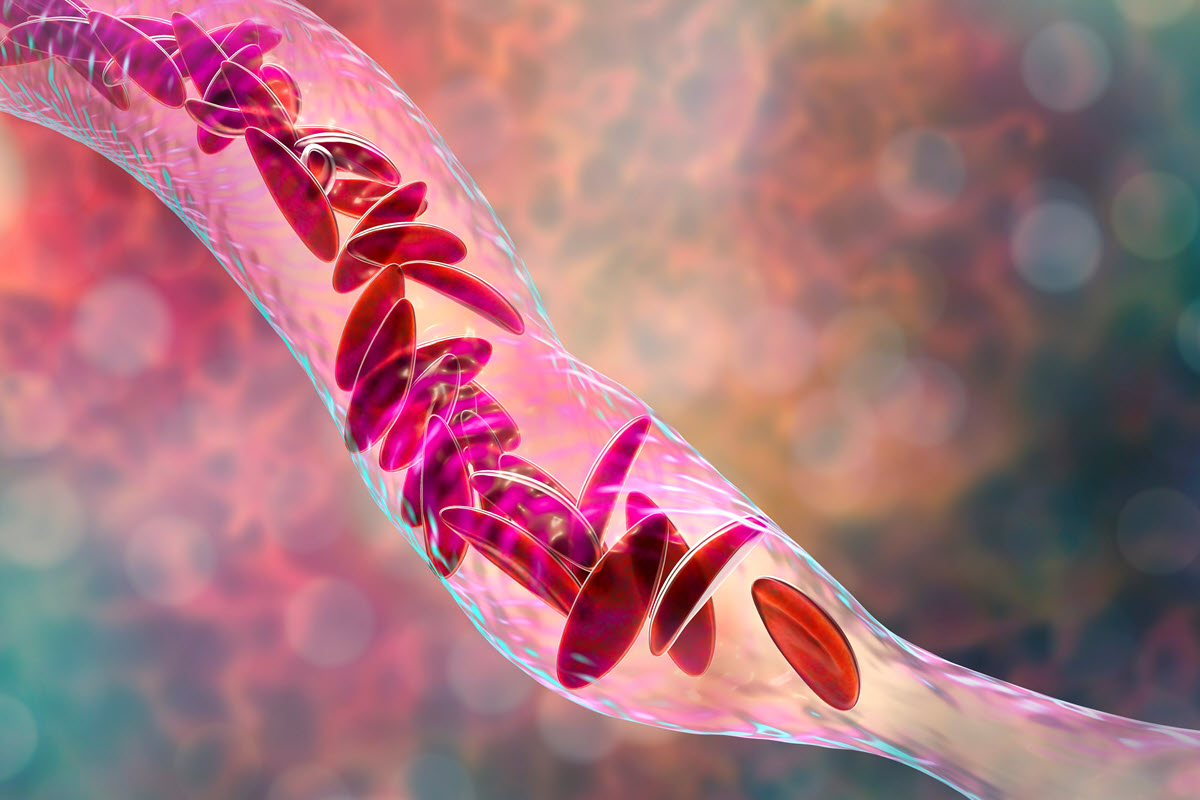
On December 8, 2023, the FDA announced its approval for two new cell-based gene therapies for the treatment of sickle cell disease (SCD), which affects approximately 100,000 people in the US, primarily those of African American descent and, to a lesser degree – Hispanic Americans.
The first gene therapy is Lyfgenia from Bluebird Bio, which uses a lentiviral vector as a gene delivery mechanism to initiate an ex-vivo (e.g. outside the body) genetic modification in the patient’s blood stems cells, causing them to produce hemoglobin HbA, which, when introduced to the patient, displace the malformed sickle-shaped Hemoglobin A blood cells created by those affected with SCD.
The second FDA-approved gene therapy is Casgevy from CRISPR Technologies and Vertex, which, as the name implies, uses CRISPR/CAS9 gene editing technology to edit the genetic structure of the patient’s blood stem cells in ex-vivo to induce the production of fetal hemoglobin (HbF) when the modified stem cells are injected into the patient. The presence of increased HbF levels prevents the deformation (sickling) of red blood cells, which would otherwise occur in untreated SCD patients.

What is Sickle Cell Disease?
Sickle cell disease is an autosomal recessive disease, e.g. a genetic condition caused when a patient inherits two copies (e.g. one from each parent) of a mutation that primarily affects the adult hemoglobin A cells responsible for red blood cell production. (Fetal blood cell production is not affected by this mutation, a point that will become important when we discuss the CRISPR/CAS9 gene therapy for SCD below.)
The SCD mutation causes the blood cells to distort into the classic crescent or “sickle” shape, which can lead to a shortened lifespan due to conditions such as chronic anemia, excess free hemoglobin in the blood plasma, liver and spleen damage, blood cell buildup in the bones, brain, and lungs, as well as chronic pain due to increased pressure on the skull and swelling in the hands and feet due to vaso-occlusion.

How Can CRISPR/CAS9 Technology Edit the Gene Structure Responsible for Sickle Cell Disease?
We mentioned that fetal hemoglobin (HbF), which is responsible for red cell production during pregnancy and the first 6 to 24 months of life, is not affected by the gene defect in adult hemoglobin (HbA) that causes sickle cell in older patients.
The approach taken by CRISPR Technologies and Vertex in their Casgevy therapy is to use CRISPR/CAS9 technology to “edit” the stem cells that create fetal hemoglobin (HbF) cells by turning off the function that disables the production of fetal hemoglobin when the patient reaches between 6 and 24 months of age. This is done by extracting stem cells from the patient’s bone marrow and editing them ex-vivo in the laboratory.
Fortunately, compared to the gene modifications that would be required in other genetic disease therapies, the gene modification required in this case is relatively simple, which likely helped convince the FDA to give their first-ever approval using the relatively new CRISPR/CAS9 gene editing technology.
Speaking to Bloomberg, CRISPER/CAS9 co-inventor and 2020 Nobel Prize winner in chemistry Jennifer Doudna said: “I’m just amazed, it’s 11 years from a fundamental discovery in science to an approved therapeutic, that’s just extraordinary speed, and it speaks to the many, many people that contributed to make that possible. “

Using CRISPR to Edit the Gene Responsible for Sickle Cell Disease is Just Part of a Difficult, Complex Treatment Program
Unfortunately, the CRISPR/CAS9 gene editing therapy component may be one of the least onerous steps for patients seeking a cure for their sickle cell disease. It’s an expensive multi-step therapy (with many parallels to chemo-based cancer treatment) that could take up to a year to complete.
Because the treatment could affect sperm in men and eggs in women, many patients will want to undergo fertility preservation before the actual SCD therapy. (A big concern is this step may not be covered by insurance.)
Next, patients will have their stem cells extracted (often requiring multiple visits). While the lab is editing their stem cells, the patients will need to undergo a chemotherapy regime to remove the defective bone marrow cells that produce mutated sickled red blood cells. This will likely require a multi-month stay in the hospital while the immune system rebuilds itself. Then the CRISPR/CAS9 edited fetal hemoglobin (HbF) cells are injected into the patient, who is monitored to confirm that they are producing sufficient amounts of red blood cells – which, if successful, will provide the patient with a permanent cure to their sickle cell disease.

Formaspace is Your Laboratory Research Partner
If you can imagine it, we can build it, at our Austin, Texas factory headquarters.
Talk to your Formaspace Sales Representative or Strategic Dealer Partner today to learn more about how we can work together to make your next construction project or remodel a success.












