Illumina and arch-rival BGI are racing to introduce lower-cost genomic sequencing machines – a move that could make the use of genomic sequencing in research and clinical testing laboratories more commonplace, helping to usher in the future of personalized medicine based on individual genetic tests.

Illumina introduces a new lower-cost $200 Genome Sequencing solution at its inaugural Illumina Genomics Forum
In September 2022, Illumina introduced its new line of genomic sequencing machines – the NovaSeq X, NovaSeq X Plus, and the FDA-registered NovaSeq 6000 Dx – at a new event hosted in the company’s hometown of San Diego.
The NovaSeq X product has created the biggest splash in the press, as Illumina claims it can deliver a full genomic sequence for as little as $200. Of course, this headline is a bit misleading – the machine alone costs $985,000, and the supporting reagents are extra, but Illumina maintains that users of the new refrigerator-sized NovaSeq X machines can achieve the average target price of $200 per genome when sequencing at large scale volumes.
According to Illumina, the NovaSeq X series is designed to handle higher volume production: the NovaSeq X Plus can sequence over 20,000 whole genomes per year, a 2.5 X throughput increase compared to earlier generation sequencers.
Among the welcome new efficiency features, Illumina says the new sequencers can use reagents that can be shipped at ambient temperatures, eliminating the extra cost and complexity of packaging chemicals in dry ice or ice packs.
While there are questions about whether most laboratories will perform enough tests to achieve the claimed $200 genome cost benchmark, these new machines do represent a significant milestone in reducing the cost of genomic sequencing.
It’s worth taking a moment to recall just how dramatically prices have fallen.
As shown in the chart published by the NIH shows above, sequencing a single genome cost around $100,000,000 as recently as 2001. Over the next seven years, prices dropped in line with predictions from Moore’s Law.
The big game changer came in 2007 with the commercial introduction of high-throughput next-generation sequencing (NGS) technologies, which drove the cost of sequencing far below what Moore’s law would have predicted – with average prices dropping to around $1000 by 2015.

Complete Genomics—a subsidiary of Chinese-owned BGI’s American business unit MGI – launches a $100 Genomic Sequencing Solution
Why is Illumina dropping prices now?
Most analysts point to increased competition from arch-rival, China-based BGI (formerly Beijing Genomics Institute) and its American subsidiary, San Jose-based Complete Genomics (which BGI acquired in 2013 and merged into its instruments manufacturing business unit MGI).
In February 2023, Complete Genomics introduced a new line of high throughput genomic sequencers at the Advances in Genome Biology and Technology (AGBT) conference.
Like Illumina, they are focused on reducing the cost of sequencing operations. Complete Genomics claims that – thanks to new efficiencies (such as running 6 tests in parallel) and significantly lower reagent costs – the new machines sequence a human genome for as little as $100.
The company maintains that the new DNBSeq-T20x2 platform can produce 22 terabytes of data per day, equivalent to sequencing 50,000 whole human genomes (at 30x coverage) per year.
The DNBSEQ class machines are based on the DNA NanoBalls (DNB) sequencing technology which Complete Genomics first introduced in 2008. This approach cleaves off small genetic base pair fragments, forming them into circular DNA nanoballs, which are then image processed through a sequencing flow cell using a patented DNA co-barcoding technology.

Other Illumina business rivals could threaten its business with lower-cost genetic sequence solutions based on novel technologies.
Illumina is also likely reducing prices in reaction to potential threats to its market share from new, lower-cost genomic sequencing technologies developed by its rivals, including Pacific Biosciences (which Illumina tried to purchase in 2021 but was blocked by the FTC) as well as Oxford Nanopore Technology, Quantapore, and Stratos.
Pacific Biosciences (also known as PacBio) specializes in “long read” genetic sequences, a capability that was missing in Illumina’s portfolio. PacBio also commercialized the single-molecule real-time (SMRT) sequencing method in 2011 that allows single DNA molecules to be processed in real-time.
Another important new emerging technology is nanopore sequencing, which has the potential to reduce the cost of RNA and DNA sequencing by eliminating the need to use PCR amplification or chemical labeling. The aptly named company Oxford Nanopore Technology recently updated its low-cost, hand-held MinION product that uses nanopore technology to analyze the genetic sequence of single molecules. The newest version, the MinION Mk1c, starts at just under $5000 and is small enough to allow researchers to sequence RNA and DNA samples when working in the field.
Another potential competitor on the horizon is US-based startup Ultima, which has promised to deliver a genomic sequencing solution for $100 per run. The company’s UG 100 sequencer does not rely on a traditional rectilinear flow channel; instead, it spins the genetic material suspended in reagents on a platter, where it is quickly read using an optical camera – much like a data CD.
BGI Settled its Patent Infringement Cases against Illumina, but its Subsidiary Business Units Now Face Trade Bans by the US Government
For years, battles over intellectual property rights and patents have roiled the genomics sequencing market.
An epic legal battle between BGI’s MGI subsidiary and Illumina was largely settled in BGI’s favor in 2022.
However, in an echo over concerns about the Chinese telecommunications giant Huawei, BGI and its various business units have fallen into the crosshairs of the Federal government in Washington, D.C.
Senators Rubio and Grassley issued an alarm in 2019 about potential threats to US national security, including possible violations of US intellectual property protections and potential violations of HIPAA privacy protections as a result of transferring the genomic data of individual Americans to China or Russia for processing. The Senators’ letter specifically called out BGI and its subsidiaries, MGI and US-based Complete Genomics.
Since that time, several BGI business units have been targets for US sanctions:
- In 2019, the Department of Commerce’s Bureau of Industry and Security (BIS) added two BGI business units to its “Entities List,” Xinjiang Silk Road BGI and Beijing Liuhe BGI, “in connection with conducting genetic analyses used to further the repression of Uyghurs and other Muslim minorities in XUAR,” e.g. the Xinjiang Uygur Autonomous Region. Companies on the Entities list are subject to a US import and export ban.
- In October 2022, the US Department of Defense (DOD) added BGI Genomics Co Ltd, which it said “runs a massive gene databank and has DNA-sequencing contracts worldwide” to its list of companies believed to be owned or controlled by the Chinese military. Inclusion on this list prevents Americans from making investments in these companies.
- On March 2, 2023, the US Commerce Department added BGI Research and BGI Tech Solutions (Hongkong) as well as BGI’s forensics subsidiary, Forensics Genomics International, to its Entities List, which bans imports and exports. According to the Commerce Department, “the addition of these entities is based upon information that indicates their collection and analysis of genetic data poses a significant risk of contributing to monitoring and surveillance by the government of China, which has been utilized in the repression of ethnic minorities in China. Information also indicates that the actions of these entities concerning the collection and analysis of genetic data present a significant risk of diversion to China’s military programs.”

So far, the Federal Government has not added BGI’s US-based Complete Genomics to the export ban, but we note that Complete Genomics executives were careful during the recent launch of their newest sequencing machines to brand the equipment as “Complete Genomics” – avoiding the MGI branding heretofore used overseas.
Cheaper genomic sequencing could open up new markets for broader use in clinical healthcare screenings and home-based diagnostics, paving the way for Personalized Medicine
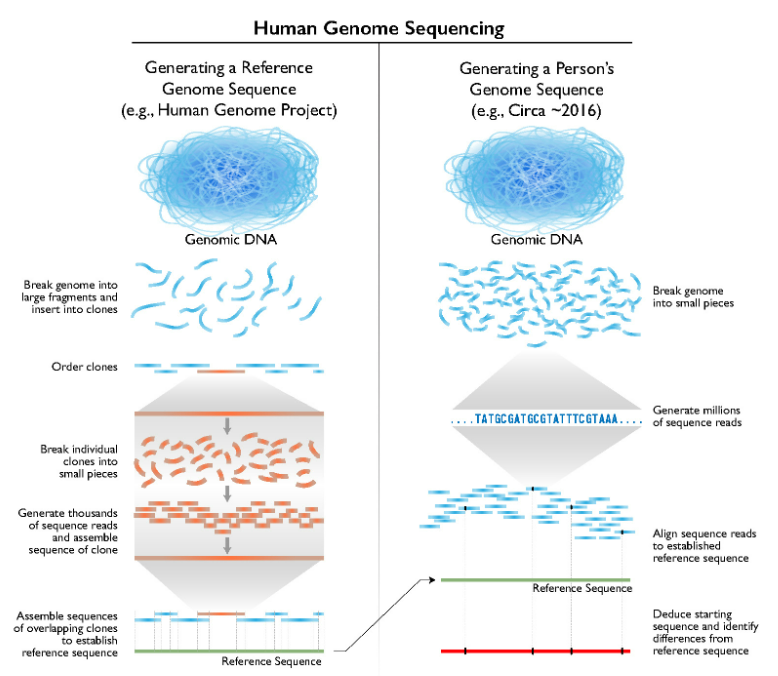
The Human Genome Project, which documented the DNA base pair sequences that make up human DNA, first popularized the concept of genetic sequencing with the public. However, it was the COVID-19 pandemic that normalized sequencing technology to where we take it for granted that new virus variants can be genetically sequenced quickly or we can depend on PCR-based tests to know whether we have been exposed to a virus.
While genomic sequencing started as a laboratory research tool, today, the majority of equipment sales are for healthcare diagnostics.

So how big is the market for genetic testing today, and how fast could it grow?
Here we’ll use the term the FDA uses to describe the market, e.g., the In Vitro Diagnostic Market (IVD), which the FDA defines as encompassing genomic sequencing testing instruments and support equipment, chemical reagents, and other related services and components.
Some industry analysts, such as AB Bernstein, estimate the total worldwide IVD market at $65 billion. In their 2021 “Blackbook,” the firm forecasted the market would grow at a 7% CAGR from 2022 to 2025.
Lower costs for genomic sequencing will lead to other important changes in the coming years:
- Less expensive genomic tests will encourage more lab researchers and clinical testing labs to incorporate genomic sequencing into their everyday research projects and clinical diagnostic testing operations.
- Smaller, cheaper instruments will help researchers and clinical healthcare providers in lower-income countries gain access to the technology, improving equitable access to healthcare around the world
Lower-cost genomic sequences could also help usher in a new era of Personalized Medicine, personalized medicine, making sequencing more accessible for mainstream healthcare to treat complex conditions, including cancer, heart disease, Alzheimer’s disease, and autoimmune disorders.
Formaspace is Your Clinical Testing and Laboratory Research Partner
If you can imagine it, we can build it, here at our Formaspace factory headquarters.
Speak with your Formaspace Design Consultant today to find out how we can work together to make your next laboratory construction or remodeling project a success.