In breaking news, the FDA just approved Moderna and Pfizer’s next-generation Covid vaccines targeting the Omicron variants BA.4/BA.5. The new vaccines are expected to become available to the public within days.
In this Formaspace.com laboratory research article, we look at the science behind Moderna and Pfizer’s next-generation Covid mRNA vaccines, including some concern about the speed of the approval process.
Looking back at our first article on Covid from March 2020 – Laboratories Around the World are Collaborating on a Coronavirus Vaccine – it’s clear how much has changed. At that time, vaccine researchers were working against a relatively “fixed” viral target, the original SARS-CoV-2 virus (today known as the “wild-type” of Covid, or WT).
However, in the two years since we wrote our first lab science article on Covid, the world has experienced successive “waves” of new Covid variants (including Alpha, Beta, Gamma, Delta, Zeta, Mu, and Omicron) that “outcompeted” viruses in circulation to become the newest dominant variant.
Of these, Omicron and its sub-variants have become the most easily transmitted – especially the sub-variant BA.5, which appears to be capable of infecting individuals even when they are socially distancing outdoors.

Why are first-generation Moderna/Pfizer mRNA Covid vaccines less effective against Omicron variants, including BA.5?
Unfortunately, these Omicron variants are not only more transmissible, but they also can create “immune escape” infections that evade the first-generation mRNA vaccines.
Why is this?
First, a little recap on the mRNA vaccine mechanism.
The first-generation mRNA vaccines from Pfizer and Moderna inject RNA molecules (encapsulated in a lipid jacket) into the body, where they enter cells and begin to rapidly replicate – creating copies that mimic the distinctive spike protein found on the surface of the original wild-type SARS-CoV-2 virus. These replicated RNA molecules generate an immune response, essentially teaching the immune system to be prepared to fight this new invader the next time it encounters it.
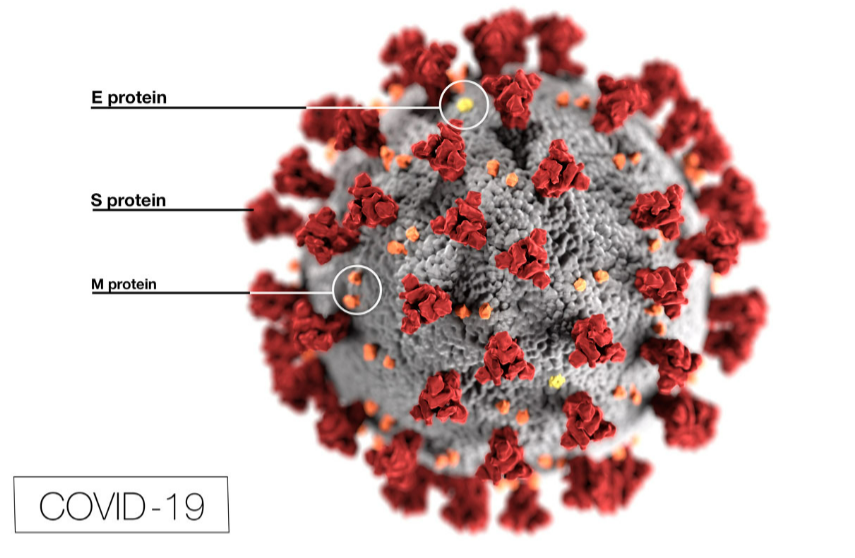
However, new Omicron sub-variants have mutated significantly from the original wild-type SARS-CoV-2 virus, especially in the spike region, rendering the original mRNA vaccines less effective in preventing infection.
A newly published paper in Science Immunology maps out the “antigenic cartography” of the different Covid variants, confirming that Omicron sub-variants BA.1 and BA.2 are significant antigenic outliers as compared to previous all variants – in other words, the obvious characteristics (such as the “spike” protein structure) have changed so much that the original first-generation mRNA vaccines are “training” the immune system to look for the wrong thing.
Another recent paper published in Nature sought to quantify the specific changes to the molecular surface of the different Omicron sub-variants. The researchers found that BA.1 variants had three mutations to the spike (L452Q, L452R, and F486V) that foiled antibodies from the mRNA vaccines or those generated naturally from previous pre-Omicron infections.

Frustratingly, researchers found the BA.4/BA.5 variants changed the game again. These variants have further mutations (S371F, D405N, and R408S) that not only evade pre-Omicron antibodies (whether naturally occurring or elucidated via vaccines) but ALSO evade antibodies generated from a naturally occurring BA.1 infection.
In other words, becoming infected with BA.1 does not appear to give people as much immune protection from a later BA.4/BA.5 infection as you might expect.
This could explain how some unfortunate individuals can experience multiple bouts of Covid – these might be caused by exposure to different variants over time. The situation is further complicated by the tendency of the human immune system to retain a high immune reaction to the very first Covid infection encountered, a phenomenon known as “original antigenic sin.”
Why have so many Covid variants arisen so quickly? The answer may be Long-Covid patients.
Why have so many Covid variants arisen so quickly?
One explanation is that this should be expected – viruses typically mutate over time to survive.
Unfortunately, one of our best hopes of preventing the emergence of dangerous mutations was to vaccinate the world’s population as fast as possible – to halt the spread of the disease before more dangerous mutations could arise. However, we have largely failed to do this in time.

While the theory that viruses in the wild will mutate on their own stands, researchers are also zeroing in on another, smaller population of patients – those who are suffering from months-long bouts of Covid disease – as a potential source of viral mutations.
Evidence is building that chronic “Long Covid” patients undergoing intensive clinical therapies might unwittingly create an environment conducive to promoting viral mutations that evade treatment measures – mimicking the tendency of bacteria to mutate and become resistant to antibiotic drugs.
How effective will the next-generation Covid vaccines be against BA.5?
Both Pfizer and Moderna have been hurrying up efforts to update their original mRNA vaccines to address these new Omicron variants.
In March 2022, Moderna published a note in the New England Journal of Medicine demonstrating test results from a new prototype bivalent vaccine dose which combines the original wild-type formula with an updated formulation targeting Omicron variant B.1.1.529.
This new vaccine produced significantly more antibodies to the Omicron variant B.1. However, the study did not evaluate the effectiveness against newer BA.4/BA.5 variants. Nevertheless, this news helped win over the UK’s MHRA (the British equivalent of the FDA), which recently approved the new bivalent Moderna vaccine for use in Britain.

Moderna then petitioned the FDA to approve this bivalent vaccine for use in America. However, the FDA decided in late June 2022 to hold off on approving it until Moderna (and Pfizer) could deliver a re-worked version that targets BA.4/BA.5 subvariants directly.
To us, this makes sense, as these are the most prevalent variants circulating at the moment. And this approach was also in line with Pfizer’s next-generation Covid vaccine development program, designed to tackle the BA.4/BA.5 variants directly.
The FDA announces approval of the next-generation Moderna and Pfizer Covid vaccines at their August 31, 2022 press conference.
In breaking news, the FDA announced today (August 31, 2022) it has approved an emergency use authorization of the new, next-generation Covid vaccines from Pfizer and Moderna.
However, there remain some ongoing concerns among medical professionals and regulatory analysts that the FDA’s approval may be too hasty.
The crux of the criticism is that this sped-up timetable (e.g. a national rollout starting in September 2022) would not allow sufficient time for clinical trials to be completed before the vaccines are deployed. Defending this approach, FDA Commissioner Dr. Robert M. Califf tweeted that they are relying on the past track record of first-generation mRNA vaccines supplemented with data collected from mice studies and other sources (such as the UK bivalent vaccine) to make the decision. We hope there are no nasty surprises down the road.
That is not the only controversy on the horizon. This past week Moderna sued Pfizer (and their vaccine developer BioNTech) for infringing on its mRNA patents filed between 2010 and 2016. It’s unlikely this development will impact the rollout of new Covid vaccines this fall, but this litigation could be very costly for Pfizer if they are found to be in the wrong.
As information becomes available, we’ll give another update on Formaspace.com explaining what we can expect from the roll-out of these two next-generation Covid vaccines in the USA, now planned to begin in early September 2022.
Stay tuned.
Formaspace is Your Partner for Laboratory Science
If you can imagine it, we can build it, here at our factory headquarters in Austin, Texas.
Speak with your Formaspace Design Consultant today and find out how we can make your next laboratory construction project or remodel a success.