We take a look at how manufacturers are heeding the call to produce more PPE equipment, hygiene products, clinical tests, and treatment therapies, including efforts to create a coronavirus vaccine.
Coronavirus Creates Unprecedented Demand for PPE, including Face Masks, Face Shields, Gloves, and Protective Gowns
It’s hard to imagine this today, but, if we were to go back in time to just a few months ago, we’d find thousands if not millions of highly sought after N95 masks sitting on warehouse shelves around the world – available for purchase for as little as $.15 each.
Fast forward to the present time. There has been a worldwide scramble to secure vital personal protective equipment (including N95 masks, face shields, gloves, and protective gowns) that are urgently needed by healthcare providers, first responders, nursing home attendants, and more in the fight against Covid-19.
The current price for an N95 mask? Anywhere from $2.50 to as much as $12 apiece, possibly more.
American manufacturers of N95 masks (including 3M, Honeywell, and Kimberly-Clark) have stepped up production dramatically. 3M has pledged to raise production to 50 million per month, but this won’t be sufficient to meet demand in the short term. Over the long term, however, 3M does hope to make more than a billion masks by the end of 2020.
As demand (and asking prices) have soared, fraud has become a rampant issue. Seemingly overnight, over 1,000 new companies registered as suppliers on the FDA’s list of manufacturers, including many non-medical factories in China, until the FDA stopped sales of defective masks that didn’t work as promised. But it turns out that fraudsters don’t need a factory to try to fleece the public: US agents cracked down on two individuals in California seeking $4 million for non-existent masks.
Fortunately, there are more of us across the supply chain trying to do the right thing.

For example, the makerspace community has stepped in to share face mask designs that can be printed on 3D printers, and a virtual army of individuals working from home has taken up sewing fabric face masks.
And a new non-profit organization, Project N95 (staffed by volunteers in the healthcare products supply chain), is working to help sort out the mess created by the chaos erupting between US states and hospital systems competing against each other to source masks and other PPE.
Many non-traditional manufacturers have quickly switched their production lines over to help solve the PPE shortage.
LVMH, the French luxury product group that owns the iconic brands Dior, Fendi, Givenchy, and Louis Vuitton, has announced it will source 40 million face masks. (LVMH is a Formaspace customer.) Other well-known fashion and clothing brands, including Prada, Gucci, and Zara, have also announced they will produce face masks. Meanwhile, in the UK, the John Lewis department store reopened a factory to produce protective gowns, and the Royal Mint, better known for producing royal collectibles, has started manufacturing face shields.

Finally, we can’t overlook the outstanding contributions made by the workers at the Braskem America plant in Neal, West Virginia. The chemical factory is a key supplier of polypropylene used to make the non-woven fabric used in face masks, medical gowns, and disinfectant wipes. In just one month, the plant produced 40 million pounds of polypropylene (the equivalent needed to make half a billion N95 masks or 1.5 billion surgical masks).
How did the Braskem workers achieve this remarkable production record? They moved from home to live inside the factory so that it could operate 24 hours a day.
Race to Supply Clinical Equipment for Covid-19 Testing, including Test Kits, Testing Reagents, and Swabs
After a rocky start dogged by defective testing kits, the US has increased the number of tests performed from a low of 2,000 in early March to around 25,000 per day currently. As of May 11, the CDC reports it has tested 6,275 specimens, and U.S. public health labs have tested 818,682 specimens. But to track and trace the virus, much more testing needs to be done – HHS testing czar Brett Giroir suggested that by September, the US will be able to test up to 50 million people per month for active Coronavirus infections.
How will we get there?

Part of the solution comes from one of Formaspace’s clients, the pharma giant Roche, which has been at the forefront of Covid-19 testing. The FDA recently approved an “emergency use authorization” for Roche’s line of Cobas PCR diagnostic testing equipment. The Cobas 6800 can complete up to 1,440 Covid-19 samples per day, while the Cobas 8800 is capable of 4,128 tests per day, providing results in as little as 4 hours.
Meanwhile, San Diego-based Quidel has introduced a new antigen test that can identify Covid-19 infections. The antigen approach has the potential to provide results more quickly and at a lower cost than the conventional PCR test. The FDA has issued also issued an “emergency use authorization” for the new Quidel system, known as Sofia 2 SARS Antigen FIA, which can analyze nasal swap samples in a doctor’s office setting and produce test results within 15 minutes.
Public health officials point out that, while it’s critical to know who has an active Covid-19 infection, it’s equally important to identify those who have been exposed to the virus — and have developed antibodies to fight off the disease.
Roche has announced the production of a new, high-volume Covid-19 antibody testing system that can identify if a patient’s blood sample has evidence of antibodies, which indicates a patient has fought off a Covid-19 viral infection. This new Elecsys® Anti-Sars-CoV-2 antibody test system, which received FDA emergency use approval, is capable of running 300 tests per hour, with single results in as little as 18 minutes. By years end, Roche expects the number of tests performed to grow from the low millions to as many as 100 million per month by the end of 2020.
Roche CEO Severin Schwan discusses their new Covid-19 antibody test solution.
Automakers Step in to Produce Vitally Needed Ventilators
As the number of coronavirus cases skyrocketed, so too did the need for ventilators used to treat patients whose lungs were under attack by the Covid-19 virus.
Traditional ventilator producers, such as Medtronic (a Formaspace customer), dramatically ramped up their ventilator production by scheduling back to back shifts working 24 hours around the clock.
Despite the increase in production at Medtronic (which rose from 40% then 200% over Nora production levels), there was widespread concern about having enough ventilators on hand to supply in the nation’s intensive care units should the number of coronavirus cases seen in the New York City area become the norm across the nation.

Seeing a potential crisis in the making, the US government’s Department of Health and Human Services stepped in and ordered 30,000 ventilators (at a cost of $489 million) from General Motors, with just over 6,000 machines to be delivered by June 1 and the balance by August. Working in partnership with Seattle area-based Ventec Life Systems, GM quickly converted one of its existing automotive component manufacturing facilities in Kokomo, Indiana to begin the urgent production of Ventec’s portable VOCSN ventilator.
GM also turned to us here at Formaspace for urgent help in converting their Kokomo, Indiana facility over to ventilator production. Working just from a verbal order over the phone, we began production of 500 new Formaspace workstations (with built-in ESD protection) — we were able to complete the order and get all 500 units loaded on trucks for Indiana in just 18 days. Remarkably, once our 1st truckload arrived in Indiana, General Motors was able to stand up an initial ventilator production line in just a few days.
General Motors and Ford, both Formaspace customers, are now assembling ventilators to meet the needs of intensive care units treating Covid-19 patients.
Non-Traditional Companies Make Hand Sanitizer
Many of us have been surprised to learn that our local breweries have begun to produce a product that been in very short supply since the start of the Covid-19 pandemic.
No, we’re not talking about beer, although beer sales have risen significantly while many of us have been under stay-at-home orders.
The answer is hand sanitizer.
It makes sense if you think about it.
Beer and spirits manufacturers are well-equipped to process the main ingredient in hand sanitizer – which is, after all, just alcohol, albeit in a non-drinkable form.
For example, the regional brewer Saint Arnold’s (based in Houston) has started hand sanitizer production, and the makers of Absolut Vodka and Jameson Irish Whiskey have converted their facilities to produce hand sanitizer – and they will donate it to where it’s needed most.
Perfume manufacturers are also getting into the act, Formaspace customer LHVM is not only making face masks (as mentioned above), its perfume division has started producing hand sanitizer as well.
Fast Track Efforts to Create Clinical Therapies and Vaccines for Covid-19
All of us look forward to the day when a vaccine becomes widely available that can halt the spread of the Covid-19 virus and bring this pandemic to an end.
In our first article on the new coronavirus that appeared in early March of this year, we identified many of the research projects underway that are actively looking for clinical therapies or outright vaccines to control Covid-19.
There have been significant developments since that article first appeared.

For example, researchers at Stanford University and the Chan Zuckerberg Bio-Hub (both Formaspace customers) have been working day and night to gather clinical data to establish the efficacy of Gilead’s antiviral drug Remdesvir as a clinical therapy for Covid-19 patients.
As a result of these investigations, the FDA approved Remdesvir for emergency use to treat patients with active Covid-19 infections, and the drug is being rolled out nationwide.
Meanwhile, four other high priority vaccine projects are making progress in the fight against the pandemic.
Massachusetts-based Moderna, working in partnership with the NIH team led by Anthony Fauci, just received fast track status from the FDA. Phase 1 trial of their vaccine candidate started on March 16. The company announced last week it would start a Phase 2 trial with 600 people and a Phase 3 trial with thousands of volunteers by early summer. Moderna’s candidate vaccine relies on injecting the body with messenger RNA (mRNA) to provoke a response from the immune system.
British pharma giant AstraZeneca, working with researchers at Oxford University, began their phase 1 clinical trial in April, testing healthy volunteers at 5 sites in the UK. Further stage trials are hoped to commence by the middle of 2020 according to AstraZeneca. This vaccine candidate had previously shown success in preventing coronavirus in rhesus monkeys.
The pharma conglomerate Pfizer is working with its German partner BioNTech to create an mRNA-based vaccine against the Covid-19 virus. According to the company, Phase 1 trials recently began in the US East Coast, and they are already planning to ramp up production to produce millions of doses by October and hundreds of millions in 2021.
The vaccine candidate under development by Inovio stands out as it is a DNA-based vaccine (rather than RNA-based). The company announced the start of its Phase 1 study at the University of Pennsylvania and in Kansas City with preliminary results projected to be available by June.
Limited space prevents us from going into details about many of the other research projects underway around the world. Hopefully, one or more of these initiatives will prove successful in identifying the vaccine to control the spread of Covid-19.
But the discovery of a vaccine is only one part of the puzzle. Ultimately, it will need to be manufactured and distributed in very high volumes with some experts estimating that deploying 300 million doses would be a good down payment in the effort to eradicate the virus around the world
Apple and Google Cooperate to Support Contract Tracing to Control the Spread of the Virus
Finally, we like to mention how the coronavirus pandemic has brought together two of the most keenly competitive adversaries in the world of technology: Apple and Google.
These two tech giants have elected to cooperate on a joint project to develop a new track and trace API to be included in future releases of their Android and iOS smartphone operating systems. It uses Bluetooth signals to log anonymized records of other phones that you may have been in close, extended contact with.
Contract tracing technology can be built to keep your personal information anonymous, but many consumers will need more information before they trust these new apps.
Engineers working for the due companies explain that their approach is designed to maximize privacy while still providing critical tracking information that can alert smartphone users that they have come in contact with someone who has come down with the Covid-19 virus.
Formaspace is Here to Help
We are open for business.
Formaspace has the production capacity in place at our factory headquarters here in Austin, Texas to expedite your order.
To give you some idea of our capabilities, we were able to produce five hundred new workbenches for General Motors’ new ventilator facility in Kokomo, Indiana in only eighteen days.
And if you need to make changes to your facility to help protect your workers on the job, here are three popular choices that many Formaspace customers are ordering for their offices, manufacturing facilities, laboratories, and distribution centers:
Protective Health Shield
The first product is a Protective Health Shield that can be mounted to new or existing workbenches and desks. Its high walls, made of durable HDPE, help protect workers on the job by creating a physical barrier between desks.
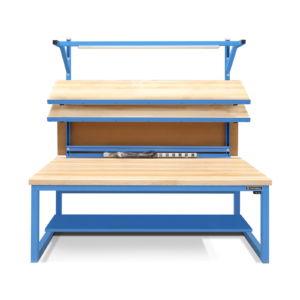
Protective Workbench Screen
The second product is a Protective Workbench Screen, custom designed for Formaspace workbenches (including Basix and Benchmarx models). This product mounts to the unistruts (upper units) of the workbench (no tools required) and provides a full-width transparent barrier.
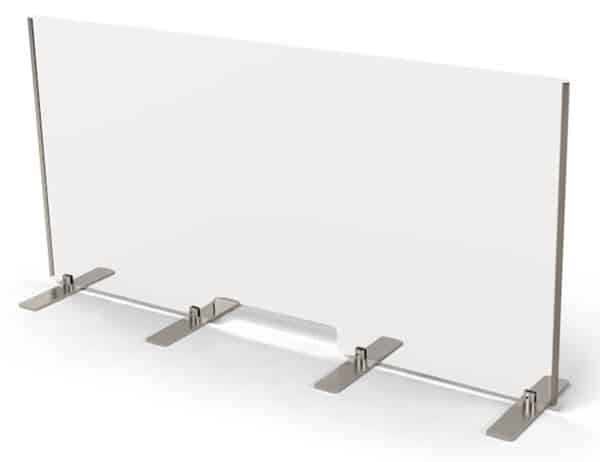
Counter Sneeze Guard
The third product is a tall transparent Counter Sneeze Guard, which is especially useful for protecting workers who need to have face-to-face interactions, such as bank tellers, cashiers, or other personnel who work directly with the public.
To find out more about these new solutions as well as our custom manufacturing capabilities, contact your Formaspace Design Consultant today.
Find out how we can work together to make your facilities safer and more efficient.